Development, Validation and Application of an ICP-SFMS Method for the Determination of Metals in Protein Powder Samples, Sourced in Ireland, with Risk Assessment for Irish Consumers
Abstract
:1. Introduction
Importance of Elemental Monitoring and Regulation in Food
2. Materials and Methods
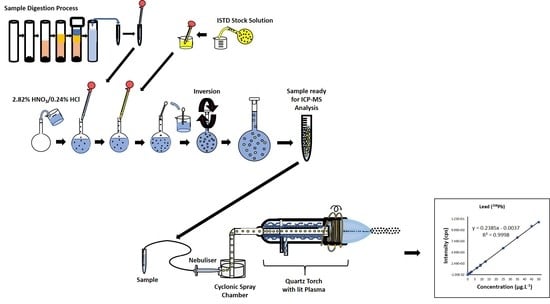
2.1. Instrumentation
2.2. Gases, Reagents and Volumetric Equipment
2.3. Quality Assurance
2.4. Sample Preparation: Microwave-Assisted Acid Digestion
2.4.1. Digestion Vessel Preparation
2.4.2. Pre-Digestion of Protein Powder Samples
2.4.3. Digestion and Preparation of Protein Powder Samples for ICP-SFMS Analysis
2.4.4. ICP-SFMS Analysis
2.5. Health Risk Assessment
2.5.1. Non-Carcinogenic Risk
2.5.2. Carcinogenic Risk
3. Results and Discussion
3.1. Concentration of Metals in Protein Powder Samples
3.2. Essential and Therapeutic Elements
3.2.1. Lithium (7Li)
3.2.2. Molybdenum (95Mo)
3.2.3. Platinum (195Pt)
3.2.4. Gold (197Au)
3.2.5. Magnesium (24Mg)
3.2.6. Vanadium (51V)
3.2.7. Chromium (52Cr)
3.2.8. Manganese (55Mn)
3.2.9. Iron (56Fe)
3.2.10. Cobalt (59Co)
3.2.11. Copper (63Cu)
3.3. Non-Essential and Potentially Toxic Elements
3.3.1. Tin (118Sn)
3.3.2. Bismuth (209Bi)
3.3.3. Titanium (47Ti)
3.4. Toxic elements
3.4.1. Beryllium (9Be)
3.4.2. Cadmium (111Cd)
3.4.3. Barium (137Ba)
3.4.4. Mercury (202Hg)
3.4.5. Thallium (205Tl)
3.4.6. Lead (208Pb)
3.4.7. Aluminium (27Al)
3.5. Health Risk Assessment
3.5.1. Non-Carcinogenic Risk
3.5.2. Carcinogenic Risk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ring, G.; O’Mullane, J.; O’Riordan, A.; Furey, A. Trace metal determination as it relates to metallosis of orthopaedic implants: Evolution and current status. Clin. Biochem. 2016, 49, 617–635. [Google Scholar] [CrossRef]
- Tedesco, R.; Hidalgo, M.D.C.V.; Vardè, M.; Kehrwald, N.M.; Barbante, C.; Cozzi, G. Trace and rare earth elements determination in milk whey from the Veneto region, Italy. Food Control 2021, 121, 107595. [Google Scholar] [CrossRef]
- Hogan, S.A.; O’Loughlin, I.B.; Kelly, P.M. Soft matter characterisation of whey protein powder systems. Int. Dairy J. 2016, 52, 1–9. [Google Scholar] [CrossRef]
- Ruano, J.; Teixeira, V.H. Prevalence of dietary supplement use by gym members in Portugal and associated factors. J. Int. Soc. Sports Nutr. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelsomin, M. The Scoop on Protein Powder. 2020. Available online: https://www.health.harvard.edu/blog/the-scoop-on-protein-powder-2020030918986 (accessed on 20 March 2021).
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Thomas, E.; Karsten, B.; Sahin, F.N.; Ertetik, G.; Martines, F.; Leonardi, V.; Paoli, A.; Gentil, P.; Palma, A.; Bianco, A. Protein supplement consumption is linked to time spent exercising and high-protein content foods: A multicentric observational study. Heliyon 2019, 5, e01508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hone, M.; Nugent, A.P.; Walton, J.; McNulty, B.A.; Egan, B.; Hone, M.; Nugent, A.P.; Walton, J.; McNulty, B.A.; Egan, B. Habitual protein intake, protein distribution patterns and dietary sources in Irish adults with stratification by sex and age. J. Hum. Nutr. Diet. 2020, 33, 465–476. [Google Scholar] [CrossRef]
- Helms, E.R.; Zinn, C.; Rowlands, D.S.; Brown, S. A Systematic review of dietary protein during caloric restriction in resistance trained lean athletes: A case for higher intakes. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 127–138. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Levkov, V.; Stafilov, T.; Pacinovski, N.; Baceva, K.; Mateva, N.; Gjorgovska, N.; Eftimova, E.; Kostadinov, T. Content of major and trace elements in raw ewes’ milk used for production of traditional white brined cheese. Slovak J. Anim. Sci. 2017, 50, 7–14. [Google Scholar]
- Khan, N.; Jeong, I.S.; Hwang, I.M.; Kim, J.S.; Choi, S.H.; Nho, E.Y.; Choi, J.Y.; Park, K.S.; Kim, K.S. Analysis of minor and trace elements in milk and yogurts by inductively coupled plasma-mass spectrometry (ICP-MS). Food Chem. 2014, 147, 220–224. [Google Scholar] [CrossRef]
- Pinto, E.; Ferreira, I.M.P.L.V.O.; Almeida, A. Essential and non-essential/toxic trace elements in whey protein supplements. J. Food Compos. Anal. 2020, 86, 103383. [Google Scholar] [CrossRef]
- Elgammal, S.M.; Khorshed, M.A.; Ismail, E.H. Determination of heavy metal content in whey protein samples from markets in Giza, Egypt, using inductively coupled plasma optical emission spectrometry and graphite furnace atomic absorption spectrometry: A probabilistic risk assessment study. J. Food Compos. Anal. 2019, 84, 103300. [Google Scholar] [CrossRef]
- Muller, E.I.; Souza, J.P.; Muller, C.C.; Muller, A.L.H.; Mello, P.A.; Bizzi, C.A. Microwave-assisted wet digestion with H2O2 at high temperature and pressure using single reaction chamber for elemental determination in milk powder by ICP-OES and ICP-MS. Talanta 2016, 156–157, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bilge, G.; Sezer, B.; Eseller, K.E.; Berberoglu, H.; Topcu, A.; Boyaci, I.H. Determination of whey adulteration in milk powder by using laser induced breakdown spectroscopy. Food Chem. 2016, 212, 183–188. [Google Scholar] [CrossRef]
- Maughan, R.J. Quality assurance issues in the use of dietary supplements, with special reference to protein supplements. J. Nutr. 2012, 143, 1843S–1847S. [Google Scholar] [CrossRef] [Green Version]
- Clean Label Project. Protein Powder—Our Point of View. 2018. Available online: https://www.cleanlabelproject.org/protein-powder-white-paper/ (accessed on 27 February 2021).
- Skibola, C.F.; Zhang, J.; Riby, J.E. Heavy metal contamination of powdered protein and botanical shake mixes. J. Environ. Health 2017, 80, 8–14. [Google Scholar]
- Rodushkin, I.; Paulukat, C.; Ponter, S.; Engström, E.; Baxter, D.C.; Sörlin, D.; Pallavicini, N.; Rodushkina, K. Application of double-focusing sector field ICP-MS for determination of ultratrace constituents in samples characterized by complex composition of the matrix. Sci. Total Environ. 2018, 622–623, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.A.R.; Sánchez, M.L.F.; Sanz-Medel, A. The potential of double focusing-ICP-MS for studying elemental distribution patterns in whole milk, skimmed milk and milk whey of different milks. Anal. Chim. Acta 2001, 442, 191–200. [Google Scholar] [CrossRef]
- Meseret, M.; Ketema, G.; Kassahun, H. Health risk assessment and determination of some heavy metals in commonly consumed traditional herbal preparations in Northeast Ethiopia. J. Chem. 2020, 2020. [Google Scholar] [CrossRef]
- Adusei-Mensah, F.; Essumang, D.K.; Agjei, R.O.; Kauhanen, J.; Tikkanen-Kaukanen, C.; Ekor, M. Heavy metal content and health risk assessment of commonly patronized herbal medicinal preparations from the Kumasi metropolis of Ghana. J. Environ. Health Sci. Eng. 2019, 17, 609–618. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Peer-Reviewed Toxicity Values (PPRTVs) Assessment. 2021. Available online: https://www.epa.gov (accessed on 20 March 2021).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profiles. 2021. Available online: https://www.atsdr.cdc.gov/ (accessed on 20 March 2021).
- Gu, S.-Y.; Shin, H.-C.; Kim, D.-J.; Park, S.U.; Kim, Y.-K. The content and health risk assessment of micro and toxic elements in cereals (oat and quinoa), legumes (lentil and chick pea), and seeds (chia, hemp, and flax). J. Food Compos. Anal. 2021, 99, 103881. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Cai, L.-M.; Hu, G.-C.; Wen, H.-H.; Luo, J.; Xu, H.-Q.; Chen, L.-G. An integrated exploration on health risk assessment quantification of potentially hazardous elements in soils from the perspective of sources. Ecotoxicol. Environ. Saf. 2021, 208, 111489. [Google Scholar] [CrossRef] [PubMed]
- Onyedikachi, U.B.; Belonwu, D.C.; Wegwu, M.O. Human health risk assessment of heavy metals in soils and commonly consumed food crops from quarry sites located at Isiagwu, Ebonyi State. Ovidius Univ. Ann. Chem. 2018, 29, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Aendo, P.; Thongyuan, S.; Songserm, T.; Tulayakul, P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci. Total Environ. 2019, 689, 215–222. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Regional Screening Table: Mid-Atlantic Risk Assessment. Available online: https://semspub.epa.gov/work/10/500011899.pdf (accessed on 20 March 2021).
- Krachler, M.; Irgolic, K.J. The potential of inductively coupled plasma mass spectrometry (ICP-MS) for the simultaneous determination of trace elements in whole blood, plasma and serum. J. Trace Elem. Med. Biol. 1999, 13, 157–169. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Lithium: Occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 2002, 21, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Belaidi, A.A. Molybdenum in Human Health and Disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 415–450. [Google Scholar]
- Russell, R. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; A Report of the Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222301/ (accessed on 14 March 2021).
- Davis, P. Gold therapy in the treatment of rheumatoid arthritis. Can. Fam. Physician 1988, 34, 445–452. [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed.; National Academies Press: Washington, DC, USA, 1989; 12, Other Substances in Food. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234937/ (accessed on 21 March 2021).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of gold (E 175) as a food additive. EFSA J. 2016, 14, 4362. [Google Scholar]
- De Baaij, J.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Durlach, J. Recommended dietary amounts of magnesium: Mg RDA. Magnes. Res. 1989, 2, 195–203. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Available online: https://www.ncbi.nlm.nih.gov/books/NBK109816/ (accessed on 21 March 2021).
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- LennTech. Recommended Daily Intake of Vitamins and Minerals. 2021. Available online: https://www.lenntech.com/recommended-daily-intake.htm (accessed on 21 March 2021).
- Mehri, A. Trace elements in human nutrition (II)—An update. Int. J. Prev. Med. 2020, 11. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- UK National Health Service (NHS). Vitamins and Minerals: B Vitamins and Folic Acid. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-b/ (accessed on 26 March 2021).
- European Food Safety Authority (EFSA). Tolerable Upper Intake Levels for Vitamins and Minerals. 2006. Available online: https://www.efsa.europa.eu/ (accessed on 26 March 2021).
- Gad, S.C. Bismuth. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 512–513. [Google Scholar]
- Sano, Y.; Satoh, H.; Chiba, M.; Okamoto, M.; Serizawa, K.; Nakashima, H.; Omae, K. Oral toxicity of bismuth in rat: Single and 28-day repeated administration studies. J. Occup. Health 2005, 47, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Tibau, A.V.; Grube, B.D.; Velez, B.J.; Vega, V.M.; Mutter, J. Titanium exposure and human health. Oral Sci. Int. 2019, 16, 15–24. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. International Agency for Research on Cancer Group 1 (Carcinogenic to Humans) and Group 2A (Probably Carcinogenic to Humans). 2019. Available online: https://www.cancer.org/ (accessed on 26 March 2021).
- Flick, D.; Kraybill, H.; Dlmitroff, J. Toxic effects of cadmium: A review. Environ. Res. 1971, 4, 71–85. [Google Scholar] [CrossRef]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.-A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Scientific Committee on Health and Environmental Risks (SCHER) Assessment of the Tolerable Daily Intake of Barium. 2012. Available online: https://ec.europa.eu/health/scientific_committees/ (accessed on 26 March 2021).
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Kemnic, T.R.; Coleman, M. Thallium Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy metals and probabilistic risk assessment via rice consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef] [PubMed]
| Group | Group Elements |
|---|---|
| Bulk Elements | C, H, N, O, Ca, P, S |
| Major Electrolytes | Cl, K, Na |
| Essential Elements | Co, Cr, Cu, F, Fe, I, Mg, Mn, Mo, Ni, Se, Si, V, Zn |
| Therapeutic Elements | Au, Br, Li, Pt |
| Non-essential/Potentially toxic | Sn, Bi, Ti, |
| Toxic Elements | Al, As, Ba, Be, Cd, Hg, Pb, Sn, Tl |
| Elements of Potential Interest | Ag, B, Ce, Cs, Ge, La, Rb, Sb, Sr, Th, U, W |
| Low/Other Elements of Potential Interest | Sc, Y, Zr, Hf, Nb, Ta, Tc, Re, Ru, Os, Rh, Ir, Pd, In, Te, lanthanides (excluding Ce), actinides (excluding Th and U), radioactive elements Tc, Fr, Ra, Ac, Po and At |
| 0.2 µg·L−1 | 1 µg·L−1 | 5 µg·L−1 | 15 µg·L−1 | 40 µg·L−1 | |
|---|---|---|---|---|---|
| %Rec ± RSD (n = 3) | %Rec ± RSD (n = 3) | %Rec ± RSD (n = 3) | %Rec ± RSD (n = 3) | %Rec ± RSD (n = 3) | |
| 7Li | 98.1 ± 18.8 * | 95.2 ± 13 | 92.7 ± 7.2 | 96.6 ± 4.6 | 99.5 ± 4 |
| 9Be | 116.6 ± 1.4 | 102.8 ± 2.1 | 101.8 ± 2.1 | 103.1 ± 3.9 | 104.6 ± 3.2 |
| 95Mo | 123.2 ± 0.9 | 101.2 ± 0.5 | 98.2 ± 0.6 | 100.4 ± 1.1 | 99.8 ± 0.4 |
| 111Cd | 111 ± 1.3 | 97.4 ± 1.1 | 96.4 ± 0.9 | 97.3 ± 1.4 | 101.1 ± 1.2 |
| 118Sn | 118.2 ± 0.5 | 105.3 ± 0.5 | 101 ± 0.5 | 104.3 ± 1.2 | 100.3 ± 0.8 |
| 137Ba | 96.6 ± 0.2 | 95.4 ± 1.5 | 111.9 ± 0.5 | 104.8 ± 1.1 | 101.2 ± 0.8 |
| 195Pt | 100.3 ± 9.9 | 97.5 ± 1.3 | 100.6 ± 0.8 | 101.4 ± 0.3 | 100.2 ± 0.3 |
| 197Au | 103.9 ± 0.8 | 93.3 ± 0.1 | 98.6 ± 0.3 | 99.4 ± 0.6 | 99.9 ± 0.7 |
| 202Hg | 87.8 ± 1.9 * | 96.9 ± 4.3 | 97.2 ± 0.3 | 99.1 ± 0.3 | 102.6 ± 0.6 |
| 205Tl | 93.5 ± 9.9 | 95.6 ± 8.9 | 94.7 ± 9 | 95.2 ± 8.2 | 94.5 ± 8.2 |
| 208Pb | 97.8 ± 1.5 | 99.9 ± 2.2 | 108.3 ± 1.2 | 103.1 ± 1.5 | 100.8 ± 1.6 |
| 209Bi | 101.2 ± 1.6 | 100.7 ± 0.9 | 100.2 ± 0.7 | 101.7 ± 1.4 | 99.7 ± 1.9 |
| 24Mg | 89.5 ± 5.3 d | 116.3 ± 3.5 | |||
| 27Al | 115 ± 12.6 c | 98.5 ± 0.0 d | 112.6 ± 3.6 | ||
| 47Ti | 105.2 ± 12.8 * | 101.3 ± 3.4 | 101.2 ± 2.2 | 101.8 ± 2.3 | 100.5 ± 0.6 |
| 51V | 95.9 ± 10.2 | 96.4 ± 5.4 | 97.1 ± 0.4 | 98.3 ± 2.5 | 98.3 ± 1 |
| 52Cr | 93.3 ± 2.4 a | 117.1 ± 3.3 | 101.4 ± 1.2 | 101.2 ± 2.1 | 99.2 ± 0.3 |
| 55Mn | 98.7 ± 1.6 a | 90.3 ± 0.1 b | 117.2 ± 0.9 | 100.8 ± 3.4 | 99.3 ± 1.7 |
| 56Fe | 109.8 ± 2.8 | 105.4 ± 2.0 | |||
| 59Co | 96.3 ± 7.9 | 96.5 ± 6.0 | 97.6 ± 1.2 | 98.3 ± 4.0 | 99.3 ± 2.8 |
| 63Cu | <LOQ | 94.7 ± 4.7 b | 94.3 ± 6.6 c | 94.3 ± 4.4 d | 91.8 ± 1.6 e |
| Sample ID | Protein Type | Mass per Serving/Scoop (g) | Sample Weight (g) | Sample ID | Protein Type | Mass per Serving/Scoop (g) | Sample Weight (g) |
|---|---|---|---|---|---|---|---|
| P1 | Mixed Plant | 60 | 0.4508 | P19 | Casein | 33 | 0.4493 |
| P2 | Pea | 30 | 0.4885 | P20 | Whey | 25 | 0.4641 |
| P3 | Soy | 28.5 | 0.4961 | P21 | Whey | 25 | 0.4423 |
| P4 | Whey | 30.4 | 0.4788 | P22 | Soy | 10 | 0.4276 |
| P5 | Whey | 25 | 0.4762 | P23 | Whey | 25 | 0.5074 |
| P6 | Whey | 25 | 0.4515 | P24 | Whey | 30 | 0.4548 |
| P7 | Whey | 25 | 0.4122 | P25 | Whey | 35 | 0.4389 |
| P8 | Whey | 25 | 0.4391 | P26 | Whey | 35 | 0.441 |
| P9 | Whey | 25 | 0.4619 | P27 | Whey | 25 | 0.4935 |
| P10 | Whey | 25 | 0.4287 | P28 | Whey | 29.4 | 0.4993 |
| P11 | Whey | 30.4 | 0.4708 | P29 | Mixed Plant | 35 | 0.4882 |
| P12 | Pea | 10 | 0.4391 | P30 | Whey | 28 | 0.4882 |
| P13 | Whey | 30.4 | 0.4524 | P31 | Whey | 25 | 0.5096 |
| P14 | Whey | 30 | 0.4701 | P32 | Whey | 28 | 0.5114 |
| P15 | Whey | 30 * | 0.4933 | P33 | Blend | 28 | 0.4136 |
| P16 | Whey | 30 | 0.4946 | P34 | Whey | 30 | 0.4803 |
| P17 | Whey | 25 | 0.4163 | P35 | Whey | 30 | 0.4139 |
| P18 | Whey | 42 | 0.4302 | P36 | Blend | 50 | 0.4746 |
| Parameter | Setting | Parameter | Setting |
|---|---|---|---|
| RF Power | 1225 W | Extraction Lens | −2000.00 V |
| Sample Gas Flow Rate * | 1.155 L·min−1 | Focus Lens * | −1320.00 V |
| Plasma Cool Gas Flow Rate | 15.5 L·min−1 | X-Deflection Lens * | −3.00 V |
| Auxiliary Gas Flow Rate | 1.3 L·min−1 | Y-Deflection Lens * | −4.95 V |
| No. of Scans/Resolution | 6 (LR), 6 (MR) | Shape Lens* | 120.00 V |
| Settling Time/Sample | 0.300 s (LR, MR) | Rotation Quadrupole 1 * | −1.25 V |
| No. of Sample per Peak/Nuclide | 10 (LR), 20 (MR) | Rotation Quadrupole 2 * | −1.05 V |
| Mass Window | 150% (LR), 125% (MR) | Focus Quadrupole 1 | −3.14 V |
| Search Window | 150% (LR), 50% (MR) | Data Acquisition Mode | Escan |
| Integration Window | 80% (LR), 60% (MR) | Washout Time | 60 s |
| Detection Mode | Pulse-counting and Analog |
| Reference Oral Dose (RfD) | Carcinogenic Slope Factor (CSF) | |||
|---|---|---|---|---|
| µg·kg−1·day−1 | Reference | (µg·kg−1·Day−1)−1 | Reference | |
| 7Li | 2.0 | [24] | - | |
| 9Be | 2.0 | [25] | 4300 | [24] |
| 95Mo | 5.0 | [24,25] | - | |
| 111Cd | 1.0 | [14,26,27] | 380.0 | [28,29] |
| 118Sn | 600.0 | [14,26] | - | |
| 137Ba | 200.0 | [25] | - | |
| 195Pt | - | - | ||
| 197Au | - | - | ||
| 202Hg | 0.3 | [14,27] | - | |
| 205Tl | 0.01 | [24] | - | |
| 208Pb | 3.5 | [27] | 8.5 | [28,29] |
| 209Bi | - | - | ||
| 24Mg | - | - | ||
| 27Al | 1000.0 | [14,26] | - | |
| 47Ti | - | - | ||
| 51V | 5.04 | [14,26,30] | - | |
| 52Cr(VI) | 3.0 | [27] | 500 | [28,29] |
| 55Mn | 140.0 | [14,23,25,26] | - | |
| 56Fe | 700.0 | [14,26] | - | |
| 59Co | 0.3 | [14,26] | - | |
| 63Cu | 40.0 | [14,26,27] | - | |
| Sample ID | 7Li | 9Be | 95Mo | 111Cd | 118Sn | 137Ba | 195Pt | 197Au | 202Hg | 205Tl | 208Pb | 209Bi | 27Al | 47Ti | 51V | 52Cr | 55Mn | 56Fe | 59Co | 63Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | µg·kg−1 | |
| P1 | <17.4 | 5.6 | 1314.7 | 58.1 | 17.7 | 1868.8 | 19.9 | 24.6 | 18.4 | 3.1 | 49.5 | 2.7 | NR* | 661.2 | 60.2 | 424.2 | NR* | NR* | 115.2 | NR* |
| P2 | 30.3 | <0.9 | 1492.6 | 40.6 | 26.7 | 319.3 | 102.7 | 10.8 | 4.3 | 0.3 | 22.2 | 0.8 | NR* | 709.0 | 40.8 | 289.2 | NR* | NR* | 188.5 | NR* |
| P3 | 90.3 | <0.9 | 868.7 | 20.7 | <17.4 | 1894.7 | 21.0 | 8.7 | 4.8 | 0.2 | 15.1 | 0.5 | NR* | 424.8 | 16.5 | 111.3 | NR* | NR* | 37.8 | NR* |
| P4 | <17.4 | <0.9 | 707.2 | 6.6 | <17.4 | 551.7 | 75.8 | 7.9 | 1.4 | 0.7 | 5.3 | 4.1 | 2068.5 | 308.4 | 2.3 | 81.4 | 149.1 | 5966.7 | 5.0 | 952.1 |
| P5 | <17.4 | <0.9 | 2165.8 | 12.5 | <17.4 | 598.0 | 68.3 | 7.8 | 1.5 | 0.0 | 2.7 | 5.9 | 1298.7 | 34.3 | 0.9 | 74.9 | 130.5 | NR* | 5.2 | 1068.0 |
| P6 | <17.4 | <0.9 | 1184.1 | 9.4 | <17.4 | 345.9 | 31.4 | 6.9 | 1.6 | 0.1 | 3.3 | 6.9 | 1525.1 | 29.7 | 2.5 | 51.4 | 153.3 | NR* | 3.3 | 905.0 |
| P7 | <17.4 | <0.9 | 1530.6 | 13.4 | <17.4 | 1326.6 | 28.0 | 7.9 | 1.9 | 1.1 | 7.8 | 8.0 | 3467.5 | 235.8 | 9.4 | 195.5 | 1909.3 | NR* | 39.4 | 3837.0 |
| P8 | <17.4 | <0.9 | 942.8 | 8.9 | <17.4 | 711.6 | 43.4 | 26.2 | 1.3 | 0.2 | 5.0 | 2.3 | 1263.7 | 30.6 | 0.6 | 58.9 | 113.7 | 7456.8 | 4.0 | 1077.9 |
| P9 | 81.0 | <0.9 | 1813.1 | 19.3 | <17.4 | 2003.6 | 52.2 | 13.4 | 4.5 | 1.8 | 5.5 | 0.6 | NR* | 688.5 | 27.4 | 469.8 | 3794.2 | NR* | 93.8 | 4381.0 |
| P10 | <17.4 | <0.9 | 1821.3 | 12.3 | <17.4 | 1409.9 | 51.6 | 9.2 | <0.2 | 1.0 | 8.6 | 3.5 | 2095.5 | 65.8 | 3.8 | 96.8 | 190.6 | NR* | 6.4 | 1266.7 |
| P11 | <17.4 | <0.9 | 219.6 | 8.6 | <17.4 | 667.7 | 43.9 | 9.9 | 2.7 | 2.0 | 5.9 | 4.2 | 6175.7 | 422.3 | 29.3 | 142.1 | 3132.3 | NR* | 39.2 | 2700.4 |
| P12 | 54.4 | 5.5 | 2348.7 | 15.4 | 329.7 | 263.7 | 43.5 | 7.4 | <0.2 | 0.3 | 22.5 | 1.0 | NR* | 677.2 | 43.5 | 210.7 | NR* | NR* | 89.5 | NR* |
| P13 | <17.4 | <0.9 | 832.8 | 14.8 | <17.4 | 1874.9 | 27.9 | 6.5 | 3.7 | 2.1 | 11.2 | 1.5 | NR* | 2042.1 | 45.7 | 234.5 | 3806.6 | NR* | 88.8 | 3268.4 |
| P14 | <17.4 | <0.9 | 669.8 | 7.1 | <17.4 | 1088.9 | 19.2 | 7.6 | <0.2 | 0.4 | 5.2 | 38.9 | 1544.2 | 69.1 | 2.7 | 77.5 | 460.1 | 6712.4 | 3.7 | 1210.8 |
| P15 | <17.4 | <0.9 | 663.7 | 10.1 | <17.4 | 1695.9 | 23.8 | 6.3 | 4.9 | 1.2 | 9.2 | 28.0 | NR* | 1131.8 | 24.9 | 247.3 | 2294.4 | NR* | 60.5 | 2615.9 |
| P16 | 34.3 | <0.9 | 1538.3 | 14.9 | <17.4 | 2388.8 | 24.0 | 6.0 | 0.8 | 1.7 | 19.3 | 3.0 | NR* | 1586.0 | 51.6 | 478.8 | 2738.6 | NR* | 59.5 | 3280.2 |
| P17 | <17.4 | <0.9 | 1797.1 | 21.0 | <17.4 | 2066.5 | 42.5 | 8.4 | 3.7 | 1.8 | 10.3 | 8.9 | NR* | 1298.9 | 30.7 | 477.9 | 3506.8 | NR* | 75.8 | 6523.8 |
| P18 | <17.4 | <0.9 | 280.8 | 14.2 | <17.4 | 1486.6 | 26.2 | 10.6 | 1.0 | 1.7 | 19.5 | 18.4 | NR* | 1228.1 | 31.0 | 627.2 | 3697.3 | NR* | 85.6 | 3944.2 |
| P19 | <17.4 | <0.9 | 546.8 | 17.0 | <17.4 | 4744.3 | 18.3 | 6.2 | 7.6 | 2.0 | 22.3 | 18.8 | NR* | 1585.5 | 51.8 | 302.9 | 4782.9 | NR* | 121.6 | 3727.2 |
| P20 | <17.4 | <0.9 | 859.1 | 13.5 | <17.4 | 2377.3 | 18.1 | 5.3 | 2.9 | 2.3 | 58.8 | 6.9 | NR* | 1753.1 | 35.9 | 604.8 | 3812.5 | NR* | 90.3 | 4717.6 |
| P21 | <17.4 | <0.9 | 1532.0 | 23.2 | <17.4 | 2241.5 | 13.2 | 7.3 | 8.1 | 1.6 | 17.6 | 5.9 | NR* | 1294.7 | 26.2 | 597.3 | 5001.5 | NR* | 95.7 | 4894.9 |
| P22 | 142.0 | <0.9 | 1223.1 | 23.4 | <17.4 | 1676.5 | 12.9 | 7.0 | 3.0 | 0.2 | 26.1 | <0.1 | NR* | 534.5 | 16.7 | 77.7 | NR* | NR* | 48.7 | NR* |
| P23 | 33.1 | <0.9 | 1320.3 | 12.3 | <17.4 | 864.0 | 16.1 | 5.8 | 1.8 | 0.6 | 3.8 | 5.0 | 3840.2 | 416.3 | 10.3 | 229.4 | 2004.6 | NR* | 46.7 | 2765.2 |
| P24 | <17.4 | <0.9 | 295.9 | 14.3 | <17.4 | 1655.5 | 17.7 | 10.3 | 1.9 | 1.5 | 17.5 | 26.9 | NR* | 1527.8 | 41.8 | 703.0 | 4153.4 | NR* | 97.1 | 4553.6 |
| P25 | <17.4 | <0.9 | 956.5 | 13.2 | <17.4 | 1156.0 | 18.3 | 12.3 | 3.8 | 0.4 | 4.4 | 5.3 | 2848.8 | 80.1 | 7.0 | 50.1 | 2487.4 | NR* | 7.1 | 3315.4 |
| P26 | <17.4 | <0.9 | 935.2 | 14.9 | <17.4 | 1388.7 | 15.7 | 5.4 | <0.2 | 0.1 | 10.8 | 8.0 | NR* | 150.7 | 6.3 | 62.2 | 4402.0 | NR* | 7.1 | 4516.8 |
| P27 | <17.4 | <0.9 | 1176.1 | 18.9 | <17.4 | 1748.8 | 18.4 | 6.3 | 1.1 | 1.7 | 14.2 | 2.3 | NR* | 985.4 | 28.2 | 442.5 | 2874.4 | NR* | 64.8 | 3110.4 |
| P28 | <17.4 | <0.9 | 1688.9 | 10.7 | <17.4 | 1254.3 | 30.7 | 8.9 | 1.4 | 1.0 | 88.4 | 3.7 | 1770.0 | 107.3 | 5.2 | 71.1 | 303.6 | NR* | 8.7 | 1171.0 |
| P29 | <17.4 | <0.9 | 727.3 | 45.1 | 30.5 | 2891.0 | 16.2 | 8.8 | 1.4 | 0.3 | 21.9 | <0.1 | NR* | 934.6 | 28.4 | 220.7 | NR* | NR* | 19.0 | 6187.1 |
| P30 | <17.4 | <0.9 | 1000.5 | 15.5 | <17.4 | 1369.4 | 13.3 | 5.6 | 0.9 | 1.2 | 7.7 | 1.5 | NR* | 683.8 | 20.9 | 283.5 | 2237.0 | NR* | 52.0 | 2464.9 |
| P31 | <17.4 | <0.9 | 100.2 | <0.61 | <17.4 | 460.9 | 12.4 | 7.7 | <0.2 | 0.3 | 0.8 | 0.7 | 1197.0 | 45.4 | <0.6 | 106.4 | 226.3 | 4190.5 | 2.5 | 573.5 |
| P32 | <17.4 | <0.9 | 1259.1 | 8.4 | <17.4 | 971.8 | 23.1 | 5.7 | <0.2 | 0.6 | 11.2 | 1.4 | 1770.7 | 149.6 | 3.8 | 99.6 | 100.8 | 6145.4 | 2.9 | 746.1 |
| P33 | <17.4 | <0.9 | 1260.1 | 12.9 | <17.4 | 1686.7 | 17.6 | 7.7 | <0.2 | 0.5 | 23.8 | 2.9 | 6356.1 | 528.9 | 12.1 | 271.1 | 2339.9 | NR* | 17.6 | 3691.6 |
| P34 | <17.4 | <0.9 | 338.0 | 15.5 | <17.4 | 2253.9 | 17.3 | 13.8 | 6.9 | 3.4 | 66.0 | 17.8 | NR* | 2313.7 | 74.1 | 481.0 | 5960.4 | NR* | 162.1 | 5654.3 |
| P35 | <17.4 | <0.9 | 266.1 | 5.4 | <17.4 | 559.1 | 15.8 | 7.5 | <0.2 | 0.0 | 3.6 | 21.3 | 1275.1 | 94.3 | 2.5 | 68.3 | 220.8 | 2550.5 | 3.7 | 664.2 |
| P36 | 63.9 | <0.9 | 957.6 | 23.1 | <17.4 | 1300.5 | 17.0 | 7.7 | <0.2 | 0.4 | 19.8 | 2.2 | NR* | 965.3 | 78.6 | 222.6 | NR* | NR* | 28.0 | NR* |
| Sample ID | Protein Powder Type | HI (All Substances) | |
|---|---|---|---|
| 1 Serving/Day | 3 Servings/Day | ||
| P1 | Mixed Plant | 0.956 | 2.868 |
| P2 | Pea | 0.448 | 1.345 |
| P3 | Soy | 0.169 | 0.508 |
| P4 | Whey | 0.123 | 0.368 |
| P5 | Whey | 0.180 | 0.540 |
| P6 | Whey | 0.108 | 0.323 |
| P7 | Whey | 0.246 | 0.739 |
| P8 | Whey | 0.101 | 0.302 |
| P9 | Whey | 0.389 | 1.166 |
| P10 | Whey | 0.195 | 0.586 |
| P11 | Whey | 0.216 | 0.647 |
| P12 | Pea | 0.141 | 0.422 |
| P13 | Whey | 0.359 | 1.077 |
| P14 | Whey | 0.105 | 0.315 |
| P15 | Whey | 0.250 | 0.750 |
| P16 | Whey | 0.362 | 1.086 |
| P17 | Whey | 0.368 | 1.105 |
| P18 | Whey | 0.403 | 1.209 |
| P19 | Casein | 0.436 | 1.307 |
| P20 | Whey | 0.322 | 0.967 |
| P21 | Whey | 0.362 | 1.085 |
| P22 | Soy | 0.078 | 0.235 |
| P23 | Whey | 0.217 | 0.651 |
| P24 | Whey | 0.307 | 0.922 |
| P25 | Whey | 0.197 | 0.592 |
| P26 | Whey | 0.194 | 0.583 |
| P27 | Whey | 0.271 | 0.814 |
| P28 | Whey | 0.228 | 0.685 |
| P29 | Mixed Plant | 0.235 | 0.704 |
| P30 | Whey | 0.243 | 0.729 |
| P31 | Whey | 0.032 | 0.095 |
| P32 | Whey | 0.146 | 0.437 |
| P33 | Blend | 0.201 | 0.602 |
| P34 | Whey | 0.520 | 1.560 |
| P35 | Whey | 0.043 | 0.129 |
| P36 | Blend | 0.295 | 0.884 |
| Sample ID | Protein Powder Type | Beryllium | Cadmium | Lead | |||
|---|---|---|---|---|---|---|---|
| 1 Serving/Day | 3 Servings/Day | 1 Serving/Day | 3 Servings/Day | 1 Serving/Day | 3 Servings/Day | ||
| P1 | Mixed Plant | 1.1 × 10−6 | 3.3 × 10−6 | 1.3 × 10−4 | 3.9 × 10−4 | 5.0 × 10−3 | 1.5 × 10−2 |
| P2 | Pea | 0.0 × 100 | 0.0 × 100 | 4.6 × 10−5 | 1.4 × 10−4 | 1.1 × 10−3 | 3.4 × 10−3 |
| P3 | Soy | 0.0 × 100 | 0.0 × 100 | 2.2 × 10−5 | 6.6 × 10−5 | 7.2 × 10−4 | 2.2 × 10−3 |
| P4 | Whey | 0.0 × 100 | 0.0 × 100 | 7.5 × 10−6 | 2.3 × 10−5 | 2.7 × 10−4 | 8.1 × 10−4 |
| P5 | Whey | 0.0 × 100 | 0.0 × 100 | 1.2 × 10−5 | 3.5 × 10−5 | 1.1 × 10−4 | 3.4 × 10−4 |
| P6 | Whey | 0.0 × 100 | 0.0 × 100 | 8.9 × 10−6 | 2.7 × 10−5 | 1.4 × 10−4 | 4.2 × 10−4 |
| P7 | Whey | 0.0 × 100 | 0.0 × 100 | 1.3 × 10−5 | 3.8 × 10−5 | 3.3 × 10−4 | 9.8 × 10−4 |
| P8 | Whey | 0.0 × 100 | 0.0 × 100 | 8.4 × 10−6 | 2.5 × 10−5 | 2.1 × 10−4 | 6.3 × 10−4 |
| P9 | Whey | 0.0 × 100 | 0.0 × 100 | 1.8 × 10−5 | 5.4 × 10−5 | 2.3 × 10−4 | 6.9 × 10−4 |
| P10 | Whey | 0.0 × 100 | 0.0 × 100 | 1.2 × 10−5 | 3.5 × 10−5 | 3.6 × 10−4 | 1.1 × 10−3 |
| P11 | Whey | 0.0 × 100 | 0.0 × 100 | 9.8 × 10−6 | 2.9 × 10−5 | 3.0 × 10−4 | 9.0 × 10−4 |
| P12 | Pea | 1.8 × 10−7 | 5.5 × 10−7 | 5.8 × 10−6 | 1.7 × 10−5 | 3.8 × 10−4 | 1.1 × 10−3 |
| P13 | Whey | 0.0 × 100 | 0.0 × 100 | 1.7 × 10−5 | 5.1 × 10−5 | 5.7 × 10−4 | 1.7 × 10−3 |
| P14 | Whey | 0.0 × 100 | 0.0 × 100 | 8.0 × 10−6 | 2.4 × 10−5 | 2.6 × 10−4 | 7.8 × 10−4 |
| P15 | Whey | 0.0 × 100 | 0.0 × 100 | 1.1 × 10−5 | 3.4 × 10−5 | 4.6 × 10−4 | 1.4 × 10−3 |
| P16 | Whey | 0.0 × 100 | 0.0 × 100 | 1.7 × 10−5 | 5.0 × 10−5 | 9.7 × 10−4 | 2.9 × 10−3 |
| P17 | Whey | 0.0 × 100 | 0.0 × 100 | 2.0 × 10−5 | 5.9 × 10−5 | 4.3 × 10−4 | 1.3 × 10−3 |
| P18 | Whey | 0.0 × 100 | 0.0 × 100 | 2.2 × 10−5 | 6.7 × 10−5 | 1.4 × 10−3 | 4.1 × 10−3 |
| P19 | Casein | 0.0 × 100 | 0.0 × 100 | 2.1 × 10−5 | 6.3 × 10−5 | 1.2 × 10−3 | 3.7 × 10−3 |
| P20 | Whey | 0.0 × 100 | 0.0 × 100 | 1.3 × 10−5 | 3.8 × 10−5 | 2.5 × 10−3 | 7.4 × 10−3 |
| P21 | Whey | 0.0 × 100 | 0.0 × 100 | 2.2 × 10−5 | 6.5 × 10−5 | 7.4 × 10−4 | 2.2 × 10−3 |
| P22 | Soy | 0.0 × 100 | 0.0 × 100 | 8.8 × 10−6 | 2.6 × 10−5 | 4.4 × 10−4 | 1.3 × 10−3 |
| P23 | Whey | 0.0 × 100 | 0.0 × 100 | 1.2 × 10−5 | 3.5 × 10−5 | 1.6 × 10−4 | 4.8 × 10−4 |
| P24 | Whey | 0.0 × 100 | 0.0 × 100 | 1.6 × 10−5 | 4.9 × 10−5 | 8.8 × 10−4 | 2.7 × 10−3 |
| P25 | Whey | 0.0 × 100 | 0.0 × 100 | 1.7 × 10−5 | 5.2 × 10−5 | 2.6 × 10−4 | 7.8 × 10−4 |
| P26 | Whey | 0.0 × 100 | 0.0 × 100 | 2.0 × 10−5 | 5.9 × 10−5 | 6.4 × 10−4 | 1.9 × 10−3 |
| P27 | Whey | 0.0 × 100 | 0.0 × 100 | 1.8 × 10−5 | 5.3 × 10−5 | 6.0 × 10−4 | 1.8 × 10−3 |
| P28 | Whey | 0.0 × 100 | 0.0 × 100 | 1.2 × 10−5 | 3.6 × 10−5 | 4.4 × 10−3 | 1.3 × 10−2 |
| P29 | Mixed Plant | 0.0 × 100 | 0.0 × 100 | 5.9 × 10−5 | 1.8 × 10−4 | 1.3 × 10−3 | 3.9 × 10−3 |
| P30 | Whey | 0.0 × 100 | 0.0 × 100 | 1.6 × 10−5 | 4.9 × 10−5 | 3.6 × 10−4 | 1.1 × 10−3 |
| P31 | Whey | 0.0 × 100 | 0.0 × 100 | 5.8 × 10−7 | 1.7 × 10−6 | 3.2 × 10−5 | 9.7 × 10−5 |
| P32 | Whey | 0.0 × 100 | 0.0 × 100 | 8.9 × 10−6 | 2.7 × 10−5 | 5.3 × 10−4 | 1.6 × 10−3 |
| P33 | Blend | 0.0 × 100 | 0.0 × 100 | 1.4 × 10−5 | 4.1 × 10−5 | 1.1 × 10−3 | 3.4 × 10−3 |
| P34 | Whey | 0.0 × 100 | 0.0 × 100 | 1.8 × 10−5 | 5.3 × 10−5 | 3.3 × 10−3 | 1.0 × 10−2 |
| P35 | Whey | 0.0 × 100 | 0.0 × 100 | 6.1 × 10−6 | 1.8 × 10−5 | 1.8 × 10−4 | 5.4 × 10−4 |
| P36 | Blend | 0.0 × 100 | 0.0 × 100 | 4.3 × 10−5 | 1.3 × 10−4 | 1.7 × 10−3 | 5.0 × 10−3 |
| Beryllium | Cadmium | Lead | ||||
|---|---|---|---|---|---|---|
| # daily servings | 1 | 3 | 1 | 3 | 1 | 3 |
| Sample ID (type) | P1 (Mixed Plant) | P1 (Mixed Plant) | P1 (Mixed Plant) | P1 (Mixed Plant) | P1 (Mixed Plant) | P1 (Mixed Plant) |
| CR value | 1.1 × 10−6 | 3.3 × 10−6 | 1.3 × 10−4 | 3.9 × 10−4 | 5.0 × 10−3 | 1.5 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ring, G.; Sheehan, A.; Lehane, M.; Furey, A. Development, Validation and Application of an ICP-SFMS Method for the Determination of Metals in Protein Powder Samples, Sourced in Ireland, with Risk Assessment for Irish Consumers. Molecules 2021, 26, 4347. https://doi.org/10.3390/molecules26144347
Ring G, Sheehan A, Lehane M, Furey A. Development, Validation and Application of an ICP-SFMS Method for the Determination of Metals in Protein Powder Samples, Sourced in Ireland, with Risk Assessment for Irish Consumers. Molecules. 2021; 26(14):4347. https://doi.org/10.3390/molecules26144347
Chicago/Turabian StyleRing, Gavin, Aisling Sheehan, Mary Lehane, and Ambrose Furey. 2021. "Development, Validation and Application of an ICP-SFMS Method for the Determination of Metals in Protein Powder Samples, Sourced in Ireland, with Risk Assessment for Irish Consumers" Molecules 26, no. 14: 4347. https://doi.org/10.3390/molecules26144347