Employment of Phenolic Compounds from Olive Vegetation Water in Broiler Chickens: Effects on Gut Microbiota and on the Shelf Life of Breast Fillets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Performance
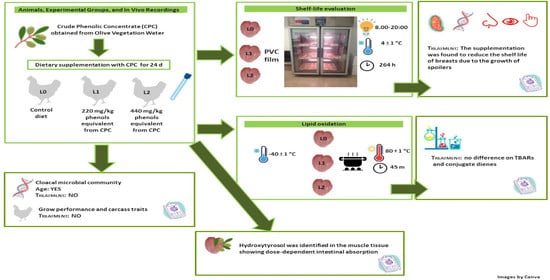
2.2. Phenol Concentration in the Diet and Breast Muscle
2.3. Gut Microbiota Profiles
2.4. Sensory Shelf Life, pH, and Drip Loss
2.5. Shelf-Life of Breast Fillets Determined with Culture-Dependent and Culture-Independent Methods
2.6. Proximate Composition, Cooking Loss, and Fatty Acid Composition
2.7. Lipid Oxidation
3. Materials and Methods
3.1. Experimental Facilities
3.2. Animals, Experimental Groups, and In Vivo Recordings
3.3. Commercial Slaughtering, and Carcass and Meat Quality Recording
3.4. Shelf-life Evaluation
3.4.1. Preparation of Packaged Chicken Breast
3.4.2. Sensory Analysis
3.4.3. Microbiological Analysis of Breast Meat during the Shelf-Life Period
3.4.4. pH and Drip Loss
3.4.5. Phenol Concentration in the Diet and in the Breast Muscle
3.5. Proximate Composition, Cooking Loss, and Fatty Acid Analysis
3.6. Lipid Oxidation
3.7. DNA Extraction and NGS Library Preparation
3.8. Statistical and Bioinformatical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Farahat, M.H.; Abdallah, F.M.; Ali, H.A.; Hernandez-Santana, A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal 2017, 11, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as Potential Attenuators of Heat Stress in Poultry Production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—Systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Y. Polyphenols in Health and Disease. Cell Biochem. Biophys. 2015, 73, 649–664. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G.F. Improvement of bioactive content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315. [Google Scholar] [CrossRef]
- Soni, M.G.; Burdock, G.A.; Christian, M.S.; Bitler, C.M.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.F.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspect of production that affect their occurence in the oil. J. Chromatgr. 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Herrero-Encinas, J.; Blanch, M.; Pastor, J.J.; Mereu, A.; Ipharraguerre, I.R.; Menoyo, D. Effects of a bioactive olive pomace extract from Olea europaea on growth performance, gut function, and intestinal microbiota in broiler chickens. Poult. Sci. 2020, 99, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Emeshili, U.K.; Iyasere, O.S.; Abioja, M.O.; Daramola, J.O.; Ladokun, A.O.; Abiona, J.A.; Williams, T.J.; Rahman, S.A.; Rotimi, S.O.; et al. Physiological responses and performance of broiler chickens offered olive leaf extract under a hot humid tropical climate. J. Appl. Poult. Res. 2017, 26, 376–382. [Google Scholar] [CrossRef]
- King, A.J.; Griffin, J.K.; Roslan, F. In vivo and in vitro addition of dried olive extract in poultry. J. Agric. Food Chem. 2014, 62, 7915–7919. [Google Scholar] [CrossRef]
- Leskovec, J.; Levart, A.; Žgur, S.; Jordan, S.; Pirman, T.; Salobir, J.; Rezar, V. Effects of olive leaf and marigold extracts on the utilization of nutrients and on bone mineralization using two different oil sources in broilers. J. Poult. Sci. 2018, 55, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Branciari, R.; Ranucci, D.; Ortenzi, R.; Roila, R.; Trabalza-Marinucci, M.; Servili, M.; Papa, P.; Galarini, R.; Valiani, A. Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter spp. Prevalence in Broiler Chickens. Sustainability 2016, 8, 837. [Google Scholar] [CrossRef] [Green Version]
- Tufarelli, V.; Laudadio, V.; Casalino, E. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ. Sci. Pollut. Res. 2016, 23, 6197–6204. [Google Scholar] [CrossRef]
- Varmaghany, S.; Rahimi, S.; Karimi Torshizi, M.A.; Lotfollahian, H.; Hassanzadeh, M. Effect of olive leaves on ascites incidence, hematological parameters and growth performance in broilers reared under standard and cold temperature conditions. Anim. Feed Sci. Technol. 2013, 185, 60–69. [Google Scholar] [CrossRef]
- Papadomichelakis, G.; Pappas, A.C.; Tsiplakou, E.; Symeon, G.K.; Sotirakoglou, K.; Mpekelis, V.; Fegeros, K.; Zervas, G. Effects of dietary dried olive pulp inclusion on growth performance and meat quality of broiler chickens. Livest. Sci. 2019, 221, 115–122. [Google Scholar] [CrossRef]
- Pečjak, M.; Levart, A.; Salobir, J.; Rezar, V. Effect of the supplementation of olive leaves and olive cake on growth performance and bone mineralisation of broiler chickens. Acta Fytotech. Zootech. 2020, 23, 105–111. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut Microbiota-Polyphenol Interactions in Chicken: A Review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Galarini, R.; Giusepponi, D.; Trabalaza-Marinucci, M.; Forte, C.; Roila, R.; Miraglia, D.; Servili, M.; Acuti, G.; Valiani, A. Oxidative status and presence of bioactive compounds in meat from chickens fed polyphenols extracted from olive oil industry waste. Sustainability 2017, 9, 1566. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J.J.; Lee, M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003, 69, 6816–6824. [Google Scholar] [CrossRef] [Green Version]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial Succession in the Broiler Gastrointestinal Tract. Appl Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Oakley, B.B.; Buhr, R.J.; Ritz, C.W.; Kiepper, B.H.; Berrang, M.E.; Seal, B.S.; Cox, N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinkowska-Lesiak, M.; Zdanowska-Sąsiadek, Z.; Stelmasiak, A.; Damaziak, K.; Michalczuk, M.; Poławska, E.; Wierzbicka, A. Effect of packaging method and cold-storage time on chicken meat quality. CyTA J. Food 2016, 14, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Raab, V.; Bruckner, S.; Beierle, E.; Kampmann, Y.; Petersen, B.; Kreyenschmidt, J. Generic model for the prediction of remaining shelf life in support of cold chain management in pork and poultry supply chain. J. Chain Netw. Sci. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Gratta, F.; Fasolato, L.; Birolo, M.; Zomeño, C.; Novelli, E.; Petracci, M.; Pascual, A.; Xiccato, G.; Trocino, A. Effect of breast myopathies on quality and microbial shelf life of broiler meat. Poult. Sci. 2019, 98, 2641–2651. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Balzan, S.; Carraro, L.; Andreani, N.A.; Taticchi, A.; Novelli, E. Application of a concentrate of phenols obtained from the vegetation water of the oil mill in the preservation of chilled food: Two case studies. Ital. J. Food. Saf. 2016, 5, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Giannenas, I.; Tzora, A.; Bonos, E.; Sarakatsianos, I.; Karamoutsios, A.; Anastasiou, I.; Skoufos, I. Effects of dietary oregano essential oil, laurel essential oil and attapulgite on chemical composition, oxidative stability, fatty acid profile and mineral content of chicken breast and thigh meat. Eur. Poult. Sci. 2016, 80, 1–18. [Google Scholar] [CrossRef]
- Starčević, K.; Krstulović, L.; Brozić, D.; Maurić, M.; Stojević, Z.; Mikulec, Z.; Bajić, M.; Mašekc, T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Moustafa, A.; Shahin, S.E.; Sherief, W.R.I.A.; Abdallah, K.; Farag, M.F.M.; Nassan, M.A.; Ibrahim, S.M. Impact of Fermented or Enzymatically Fermented Dried Olive Pomace on Growth, Expression of Digestive Enzyme and Glucose Transporter Genes, Oxidative Stability of Frozen Meat, and Economic Efficiency of Broiler Chickens. Front. Vet. Sci. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Valenzuela, R.; Echeverria, F.; Ortiz, M.; Rincón-Cervera, M.A.; Espinosa, A.; Hernandez-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, A.; Akimoto, K.; Shinmen, Y.; Kawashima, K.; Sugano, M.; Yamada, H. Sesamin is a Potent and Specific Inhibitor of Δ5 Desaturase in Polyunsaturated Fatty Acid Biosynthesis. Lipids 1991, 26, 512–516. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Miraglia, D.; Ranucci, D.; Forte, C.; Trabalza-Marinucci, M.; Servili, M.; Codini, M.; Branciari, R. Olive mill wastewater phenolic concentrate as natural antioxidant against lipid-protein oxidative deterioration in chicken meat during storage. Ital. J. Food Saf. 2018, 7, 7342. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, K.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Radić, K.; Vinković Vrček, I.; Pavičić, I.; Vitali Čepo, D. Cellular Antioxidant Activity of Olive Pomace Extracts: Impact of Gastrointestinal Digestion and Cyclodextrin Encapsulation. Molecules 2020, 50, 27. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Uk Ahn, D. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- EU Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance OJ L 276, 20.10.2010. 2010, pp. 33–79. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 26 June 2019).
- Council Regulation (EC) No 1/2005 of 22 December 2004 on the Protection of Animals during Transport and Related Operations and Amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97. OJ L 3, 5.1.2005. 2005, pp. 1–14. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02005R0001-20191214 (accessed on 14 December 2019).
- Uijttenboogaart, T.G.; Gerrits, A.R. Methods of Dissection of Broiler Carcasses and Description of Parts; Spelderholt Report 370; Spelderholt: Beekbergen, The Netherlands, 1982. [Google Scholar]
- Petracci, M.; Baéza, E. Harmonization of methodologies for the assessment of poultry meat quality features. World Poult. Sci. J. 2011, 67, 137–153. [Google Scholar] [CrossRef]
- Lanza, I.; Conficoni, D.; Balzan, S.; Cullere, M.; Fasolato, L.; Serva, L.; Contiero, B.; Trocino, A.; Marchesini, G.; Xiccato, G.; et al. Assessment of chicken breast shelf life based on bench-top and portable NIR spectroscopy tools coupled with chemometrics. Food Qual. Saf. 2020, 5, fyaa032. [Google Scholar] [CrossRef]
- Honikel, K.O.; Kim, C.J.; Hamm, R.; Roncales, P. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 1986, 16, 267–282. [Google Scholar] [CrossRef]
- Selvaggini, R.; Esposto, S.; Taticchi, A.; Urbani, S.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Optimization of the temperature and oxygen concentration conditions in the malaxation during the oil mechanical extraction process of four Italian olive cultivars. J. Agric. Food Chem. 2014, 62, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Agricultural Chemists. Official Methods of Analysis, 17th ed.; Official Methods of Analysis of AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Boselli, E.; Velazco, V.; Caboni, M.F.; Lercker, G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J. Chromatogr. A. 2001, 917, 239–244. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2015/1833 of 12 October 2015 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. OJ L 266, 13.10.2015. 2015, pp. 29–52. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2015.266.01.0029.01.ENG&toc=OJ:L:2015:266:TOC (accessed on 13 October 2015).
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid-peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- SAS Institute. The SAS/STAT 9.4 User’s Guide; SAS Inst. Inc.: Cary, NC, USA, 2013. [Google Scholar]
- LAB Fit Curve Fitting Software (Nonlinear Regression and Treatment of Data Program), v. 7.2.47. Wilton and Cleide Pereira da Silva-DF/CCT/UFPB: Brazil, 1999–2010. Available online: www.labfit.net (accessed on 5 September 2020).
- NPC Test R10. Available online: http://www.wiley.com/legacy/wileychi/pesarin/material.html (accessed on 28 March 2020).
- Baranyi, J.; Tamplin, M. ComBase: A common database on microbial responses to food environments. J. Food Prot. 2004, 67, 1834–1840. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Rukchon, C.A.; Nopwinyuwong, S.; Trevanich, T.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef] [Green Version]
- IBM Corp. IBM SPSS Statistics for Windows; Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]



| Diets | p-Value | SDR | |||
|---|---|---|---|---|---|
| L0 | L1 | L2 | |||
| Broilers, n | 40 | 40 | 39 | ||
| Live weight, g | |||||
| 1 d | 54 | 55 | 54 | n.s. | 4 |
| 24 d | 1275 | 1302 | 1304 | n.s. | 32 |
| 48 d | 3613 | 3650 | 3615 | n.s. | 230 |
| First period (1–23 d) 1 | |||||
| Daily weight gain, g/d | 53.1 | 54.2 | 54.3 | n.s. | 4.5 |
| Daily feed intake, g/d | 67.1 | 67.1 | 65.6 | n.s. | 2.5 |
| Feed conversion | 1.27 | 1.23 | 1.22 | n.s. | 0.05 |
| Second period (24–48 d) 2 | |||||
| Daily weight gain, g/d | 97.4 | 97.9 | 96.3 | n.s. | 9.0 |
| Daily feed intake, g/d | 193 | 189 | 190 | n.s. | 4.9 |
| Feed conversion | 1.98 | 1.94 | 1.98 | n.s. | 0.06 |
| Diets | p-Value | SDR | |||
|---|---|---|---|---|---|
| L0 | L1 | L2 | |||
| Broilers, n | 40 | 40 | 39 | ||
| Slaughter live weight, g | 3613 | 3650 | 3615 | n.s. | 230 |
| Cold carcass, g | 2856 | 2863 | 2845 | n.s. | 189 |
| Dressing out percentage, % | 78.8 | 78.2 | 78.5 | n.s. | 1.7 |
| Hours | Diet | |||
|---|---|---|---|---|
| L0 | L1 | L2 | ||
| 3,4-DHPEA | 24 | 78.0 ± 5.4 | 185.5 ± 8.5 | 268.5 ± 7.7 |
| 264 | <LOD | <LOD | 76.5 ± 4.0 | |
| Growth Curve Parameters | |||||
|---|---|---|---|---|---|
| Target | i | Lag | Log | Plateau | Estimated Shelf-Life (h) |
| log10 (CFU/g) | H | log10 (CFU/g/h) | log10 (CFU/g) | ||
| Total viable count ** | |||||
| L0 | 3.9 ± 0.2 | 179.1 ± 12.0 | 0.05 ± 0.02 | 7.4 ± 0.3 | 240 |
| L1 | 3.6 ± 0.3 | 120.7 ± 23.7 | 0.03 ± 0.01 | 7.8 * | 235 |
| L2 | 3.8 ± 0.2 | 169.6 ± 7.7 | 0.07 ± 0.01 | 7.9 ± 0.3 | 214 |
| Pseudomonas *** | |||||
| L0 | 1.7 ± 0.3 | - | 0.02 ± 0.003 | 7.9 ± 3.3 | 264 |
| L1 | 2.2 ± 0.2 | 71.9 ± 14.4 | 0.03 ± 0.003 | 8.3 ± 0.8 | 230 |
| L2 | 2.2 ± 0.2 | 89.9 ± 14.9 | 0.03 ± 0.003 | 8.2 * | 230 |
| Diet (D) 1 | Treatment (T) 2 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L0 | L1 | L2 | Raw | Cooked | D | T | D × T | SEM | |
| Breast (n) | 16 | 16 | 16 | 24 | 24 | ||||
| Water (%) | 72.5 | 72.7 | 72.6 | 74.9 | 70.2 | n.s | <0.001 | n.s | 0.17 |
| Crude protein (% FM) | 24.2 | 24.0 | 23.9 | 21.8 | 26.3 | n.s | <0.001 | n.s | 0.17 |
| Crude fat (% FM) | 1.48 | 1.44 | 1.60 | 1.39 | 1.63 | n.s | <0.05 | n.s | 0.050 |
| Ash (%) | 1.20 | 1.24 | 1.14 | 1.21 | 1.17 | n.s | n.s. | n.s | 0.026 |
| Crude protein (% DM) | 88.0 | 87.8 | 87.1 | 87.1 | 88.2 | n.s | n.s. | n.s | 0.37 |
| Crude fat (% DM) | 5.4 | 5.3 | 5.8 | 5.5 | 5.5 | n.s | n.s. | n.s | 0.18 |
| Ash (% DM) | 4.2 | 4.6 | 4.2 | 4.8 | 3.8 | n.s | <0.001 | n.s | 1.12 |
| Cooking loss (%) | 23.7 | 18.7 | 23.2 | n.s | 1.04 | ||||
| Diet (D) 1 | Treatment (T) 2 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L0 | L1 | L2 | Raw | Cooked | D | T | D × T | SEM | |
| Breast (n) | 16 | 16 | 16 | 24 | 24 | ||||
| C12:0 | 0.06 | 0.07 | 0.08 | 0.07 | 0.07 | n.s. | n.s. | n.s. | 0.006 |
| C14:0 | 0.86 | 0.93 | 1.00 | 0.89 | 0.97 | n.s. | n.s. | n.s. | 0.033 |
| C15:0 | 0.11b | 0.10b | 0.13a | 0.09 | 0.14 | <0.05 | <0.001 | n.s | 0.005 |
| C16:0 | 24.1 | 23.9 | 23.8 | 24.1 | 23.8 | n.s | n.s | n.s | 0.145 |
| C17:0 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | n.s | n.s | n.s | 0.004 |
| C18:0 | 8.9 | 8.8 | 8.5 | 8.8 | 8.7 | n.s | n.s | n.s | 0.122 |
| C24:0 | 1.03 | 0.93 | 0.83 | 0.90 | 0.96 | n.s | n.s | n.s | 0.040 |
| Saturates | 35.4 | 35.0 | 34.6 | 35.1 | 34.9 | n.s | n.s | n.s | 0.187 |
| C14:1 | 0.18 | 0.17 | 0.18 | 0.17 | 0.18 | n.s | n.s | n.s | 0.004 |
| C16:1 | 4.2 | 4.0 | 4.2 | 4.2 | 4.1 | n.s | n.s | n.s | 0.096 |
| C17:1 | 0.37 | 0.42 | 0.36 | 0.37 | 0.40 | n.s | n.s | n.s | 0.019 |
| C18:1 | 34.3 | 34.2 | 35,1 | 34.9 | 34.2 | n.s | n.s | n.s | 0.251 |
| C20:1 | 0.07 | 0.08 | 0.09 | 0.07 | 0.09 | n.s | n.s | n.s | 0.005 |
| Monounsaturates | 39.2 | 38.9 | 40.0 | 39.7 | 39.0 | n.s | n.s | n.s. | 0.321 |
| C18:2 n6 | 17.9b | 19.0a | 18.5ab | 18.2 | 18.6 | <0.05 | n.s | n.s | 0.148 |
| C18:3 n6 | 0.22c | 0.24b | 0.27a | 0.24 | 0.25 | <0.05 | n.s | n.s | 0.007 |
| C18:3 n3 | 1.03c | 1.11b | 1.21a | 1.05 | 1.19 | <0.001 | <0.001 | n.s | 0.018 |
| C20:2 n6 | 0.49 | 0.52 | 0.45 | 0.49 | 0.49 | n.s | n.s | n.s | 0.019 |
| C20:3 n6 | 0.74 | 0.67 | 0.66 | 0.64 | 0.74 | n.s | n.s | n.s | 0.024 |
| C20:3 n3 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | n.s | n.s | n.s | 0.002 |
| C20:4 n6 | 3.7 | 3.3 | 3.1 | 3.3 | 3.5 | n.s | n.s | n.s | 0.128 |
| C20:5 n3 | 0,17 | 0.17 | 0.15 | 0.16 | 0.16 | n.s. | n.s. | n.s. | 0.006 |
| C22:5 n3 | 0.61 | 0.57 | 0.54 | 0.55 | 0.60 | n.s. | n.s. | n.s. | 0.025 |
| C22:6 n3 | 0.37 | 0.32 | 0.29 | 0.32 | 0.33 | n.s. | n.s. | n.s. | 0.015 |
| Polyunsaturates | 25.3 | 26.0 | 25.2 | 25.0 | 26.0 | n.s. | n.s. | n.s. | 0.277 |
| LCPUFA | 6.2 | 5.7 | 5.2 | 5.5 | 5.9 | n.s. | n.s. | n.s. | 0.204 |
| n6 | 23.1 | 23.7 | 23.0 | 22.9 | 23.6 | n.s. | n.s. | n.s. | 0.242 |
| n3 | 2.2 | 2.2 | 2.3 | 2.1 | 2.3 | n.s. | <0.05 | n.s. | 0.044 |
| n6/n3 | 10.5 | 10.8 | 10.3 | 10.8 | 10.2 | n.s. | <0.05 | n.s. | 0.133 |
| Diet (D) 1 | Treatment (T) 2 | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L0 | L1 | L2 | Raw | Cooked | D | T | D × T | SEM | |
| Breast (n) | 16 | 16 | 16 | 24 | 24 | ||||
| TBARs | 0.048 | 0.048 | 0.058 | 0.034 | 0.068 | n.s. | <0.001 | n.s. | 0.005 |
| D232 | 11.4 | 11.3 | 10.8 | 11.7 | 10.7 | n.s. | <0.01 | n.s. | 0.178 |
| D270 | 7.6 | 7.9 | 7.1 | 7.6 | 7.4 | n.s. | n.s. | n.s. | 0.223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzan, S.; Cardazzo, B.; Novelli, E.; Carraro, L.; Fontana, F.; Currò, S.; Laghetto, M.; Trocino, A.; Xiccato, G.; Taticchi, A.; et al. Employment of Phenolic Compounds from Olive Vegetation Water in Broiler Chickens: Effects on Gut Microbiota and on the Shelf Life of Breast Fillets. Molecules 2021, 26, 4307. https://doi.org/10.3390/molecules26144307
Balzan S, Cardazzo B, Novelli E, Carraro L, Fontana F, Currò S, Laghetto M, Trocino A, Xiccato G, Taticchi A, et al. Employment of Phenolic Compounds from Olive Vegetation Water in Broiler Chickens: Effects on Gut Microbiota and on the Shelf Life of Breast Fillets. Molecules. 2021; 26(14):4307. https://doi.org/10.3390/molecules26144307
Chicago/Turabian StyleBalzan, Stefania, Barbara Cardazzo, Enrico Novelli, Lisa Carraro, Federico Fontana, Sarah Currò, Matteo Laghetto, Angela Trocino, Gerolamo Xiccato, Agnese Taticchi, and et al. 2021. "Employment of Phenolic Compounds from Olive Vegetation Water in Broiler Chickens: Effects on Gut Microbiota and on the Shelf Life of Breast Fillets" Molecules 26, no. 14: 4307. https://doi.org/10.3390/molecules26144307