VOCs Are Relevant Biomarkers of Elicitor-Induced Defences in Grapevine
Abstract
:1. Introduction
2. Results and Discussion
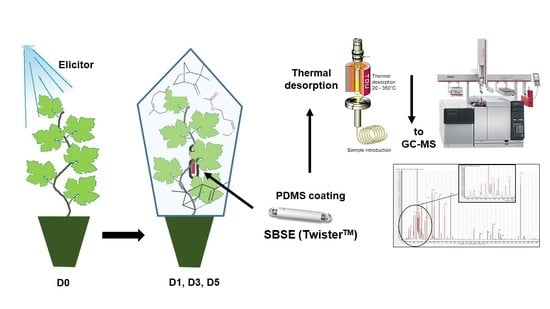
2.1. VOC Sampling Method
2.2. SBSE-GC-MS Analysis of VOC Emission Elicited by Bastid®
2.3. Comparison of VOC Emission Elicited by Other Elicitors
2.4. Elicitor-Induced Resistance Against Grapevine Downy Mildew
2.5. Analysis of Stilbenes in Bastid®- and Chitosan-Treated Grapevine Plants
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Elicitor Treatments
4.3. Induced Resistance Assays
4.4. VOC Collection and Analysis
4.4.1. Sensors
4.4.2. Containments
4.4.3. Chemicals
4.4.4. VOC Analysis by GC-MS
4.4.5. GC-MS Data Processing and Statistical Analysis
4.5. Analysis of Farnesene Synthase (VvFAR) Gene Expression
4.6. Stilbene Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heloir, M.C.; Adrian, M.; Brule, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaitre-Guillier, C.; Negrel, J.; et al. Recognition of elicitors in grapevine: From MAMP and DAMP perception to induced resistance. Front. Plant Sci. 2019, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, A.; Saigne, C.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.F.; Mérillon, J.M. Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J. Agric. Food Chem. 2006, 54, 9119–9125. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Walters, D.R.; Havis, N.D.; Paterson, L.; Taylor, J.; Walsh, D.J.; Sablou, C. Control of foliar pathogens of spring barley using a combination of resistance elicitors. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Chalfoun, N.R.; Durman, S.B.; Gonzalez-Montaner, J.; Reznikov, S.; De Lisi, V.; Gonzalez, V.; Moretti, E.R.; Devani, M.R.; Ploper, L.D.; Castagnaro, A.P.; et al. Elicitor-based biostimulant PSP1 protects Soybean against late season diseases in field trials. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clement, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clement, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bezier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Steimetz, E.; Trouvelot, S.; Gindro, K.; Bordier, A.; Poinssot, B.; Adrian, M.; Daire, X. Influence of leaf age on induced resistance in grapevine against Plasmopara viticola. Physiol. Mol. Plant Pathol. 2012, 79, 89–96. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana. Phytochemistry 2006, 67, 1520–1529. [Google Scholar] [CrossRef]
- Yi, H.S.; Heil, M.; Adame-Alvarez, R.M.; Ballhorn, D.J.; Ryu, C.M. Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiol. 2009, 151, 2152–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedlmeier, M.; Ghirardo, A.; Wenig, M.; Knappe, C.; Koch, K.; Georgii, E.; Dey, S.; Parker, J.E.; Schnitzler, J.P.; Vlot, A.C. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 2017, 29, 1440–1459. [Google Scholar] [CrossRef] [Green Version]
- Lazazzara, V.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Downy mildew symptoms on grapevines can be reduced by volatile organic compounds of resistant genotypes. Sci. Rep. 2018, 8, 1618. [Google Scholar] [CrossRef] [Green Version]
- Obara, N.; Hasegawa, M.; Kodama, O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci. Biotechnol. Biochem. 2002, 66, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, K. Age-dependent variations of volatile emissions and inhibitory activity toward Botrytis cinerea and Fusarium oxysporum in tomato leaves treated with chitosan oligosaccharide. J. Plant Biol. 2009, 52, 332–339. [Google Scholar] [CrossRef]
- Chalal, M.; Winkler, J.B.; Gourrat, K.; Trouvelot, S.; Adrian, M.; Schnitzler, J.P.; Jamois, F.; Daire, X. Sesquiterpene volatile organic compounds (VOCs) are markers of elicitation by sulfated laminarine in grapevine. Front. Plant Sci. 2015, 6, 350. [Google Scholar] [CrossRef] [Green Version]
- Van Bramer, S.; Goodrich, K.R. Determination of plant volatiles using Solid Phase Microextraction GC–MS. J. Chem. Educ. 2015, 92, 916–919. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Boland, A.; Cambier, P.; Frettinger, P.; Van Cutsem, P. Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 2010, 20, 775–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Aubel, G.; Cambier, P.; Dieu, M.; Van Cutsem, P. Plant immunity induced by COS-OGA elicitor is a cumulative process that involves salicylic acid. Plant Sci. 2016, 247, 60–70. [Google Scholar] [CrossRef]
- Clinckemaillie, A.; Decroës, A.; van Aubel, G.; Carrola dos Santos, S.; Renard, M.E.; Van Cutsem, P.; Legrève, A. The novel elicitor COS-OGA enhances potato resistance to late blight. Plant Pathol. 2017, 66, 818–825. [Google Scholar] [CrossRef]
- Wang, R.; Shen, X.; Wang, C.; Ge, R.; Zhang, Z.; Guo, X. Analysis of leaf volatiles of crabapple (Malus sp.) individuals in different aphids’ resistance. Am. J. Plant Sci. 2014, 5, 3295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Jin, Y.; Chen, H.; Wu, X. Selectivity mechanism of Anoplophora glabripennis on four different species of maples. Front. Biol. China 2008, 3, 78. [Google Scholar] [CrossRef]
- Jansen, R.M.C.; Wildt, J.; Kappers, I.F.; Bouwmeester, H.J.; Hofstee, J.W.; van Henten, E.J. Detection of diseased plants by analysis of volatile organic compound emission. Ann. Rev. Phytopathol. 2011, 49, 157–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdziej, A.; Bellee, A.; Bodin, E.; Valls Fonayet, J.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.F. Three types of elicitors induce grapevine resistance against downy mildew via common and specific immune responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef]
- Daniel, R.; Guest, D. Defence responses induced by potassium phosphonate in Phytophthora palmivora-challenged Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 2005, 67, 194–201. [Google Scholar] [CrossRef]
- De Miccolis, A.; Rotolo, R.M.C.; Gerin, D.; Abate, D.; Pollastro, S.; Faretra, F. Global transcriptome analysis and differentially expressed genes in grapevine after application of the yeast-derived defense inducer cerevisane. Pest. Manag. Sci. 2019, 75, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Brule, D.; Villano, C.; Davies, L.J.; Trda, L.; Claverie, J.; Heloir, M.C.; Chiltz, A.; Adrian, M.; Darblade, B.; Tornero, P.; et al. The grapevine (Vitis vinifera) LysM receptor kinases VvLYK1-1 and VvLYK1-2 mediate chitooligosaccharide-triggered immunity. Plant Biotechnol. J. 2019, 17, 812–825. [Google Scholar] [CrossRef] [Green Version]
- Allègre, M.; Héloir, M.-C.; Trouvelot, S.; Daire, X.; Pugin, A.; Wendehenne, D.; Adrian, M. Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol. Plant-Microbe Interact. 2009, 22, 977–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyzaniak, Y.; Negrel, J.; Lemaitre-Guillier, C.; Clement, G.; Mouille, G.; Klinguer, A.; Trouvelot, S.; Heloir, M.C.; Adrian, M. Combined enzymatic and metabolic analysis of grapevine cell responses to elicitors. Plant Physiol. Biochem. 2018, 123, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Heloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trda, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.-S.; Yang, J.W.; Choi, H.K.; Ghim, S.-Y.; Ryu, C.-M. Benzothiadiazole-elicited defense priming and systemic acquired resistance against bacterial and viral pathogens of pepper under field conditions. Plant. Biotechnol. Rep. 2012, 6, 373–380. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Li, Y.; Dai, Z. Identification of the aroma compounds in stinky mandarin fish (Siniperca chuatsi) and comparison of volatiles during fermentation and storage. Int. J. Food Sci.Tech. 2013, 48, 2429–2437. [Google Scholar] [CrossRef]
- Meng, J.; Fang, Y.; Gao, J.; Zhang, A.; Liu, J.; Guo, Z.; Zhang, Z.; Li, H. Changes in aromatic compounds of cabernet sauvignon wines during ageing in stainless steel tanks. Afr. J. Biotechnol. 2011, 10, 11640–11647. [Google Scholar]
- Zheng, J.; Zhang, F.; Zhou, C.; Lin, M.; Kan, J. Comparison of flavor compounds in fresh and pickled bamboo shoots by GC-MS and GC-olfactometry. Food Sci. Technol. Res. 2014, 20, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Altaee, N.; Kadhim, M.J.; Hameed, I.H. Detection of volatile compounds produced by Pseudomonas aeruginosa isolated from UTI patients by gas chromatography-mass spectrometry. Int. J. Curr. Pharm. Rev. Res. 2017, 7, 8–24. [Google Scholar]
- Xu, F.; Tao, W.-y.; Sun, J. Identification of volatile compounds released by myxobacteria Sorangium cellulosum AHB103-1. Afr. J. Microbiol. Res. 2011, 5, 353–358. [Google Scholar]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-beta-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Potter, D.A.; Hamilton-Kemp, T.R.; Byers, M.E. Diurnal emission of volatile compounds by Japanese beetle-damaged grape leaves. Phytochemistry 1997, 45, 919–923. [Google Scholar] [CrossRef]
- Krugner, R.; Wallis, C.M.; Walse, S.S. Attraction of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae) to synthetic formulation of a (E)-β-ocimene and (E,E)-α-farnesene mixture. Biol. Control 2014, 77, 23–28. [Google Scholar] [CrossRef]
- Ricciardi, V.; Marcianò, D.; Sargolzaei, M.; Maddalena, G.; Maghradze, D.; Tirelli, A.; Casati, P.; Bianco, P.A.; Failla, O.; Fracassetti, D.; et al. From plant resistance response to the discovery of antimicrobial compounds: The role of volatile organic compounds (VOCs) in grapevine downy mildew infection. Plant Physiol. Biochem. 2021, 160, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Krzyzaniak, Y.; Gauvrit, C.; Jamois, F.; Domergue, F.; Joubes, J.; Ferrieres, V.; Adrian, M.; Legentil, L.; Daire, X.; et al. An ethoxylated surfactant enhances the penetration of the sulfated laminarin through leaf cuticle and stomata, leading to increased induced resistance against grapevine downy mildew. Physiol. Plant. 2016, 156, 338–350. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Trouvelot, S.; Varnier, A.L.; Allegre, M.; Mercier, L.; Baillieul, F.; Arnould, C.; Gianinazzi-Pearson, V.; Klarzynski, O.; Joubert, J.M.; Pugin, A.; et al. A beta-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Mol. Plant Microbe Interact. 2008, 21, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim Khiook, I.L.; Schneider, C.; Heloir, M.C.; Bois, B.; Daire, X.; Adrian, M.; Trouvelot, S. Image analysis methods for assessment of H2O2 production and Plasmopara viticola development in grapevine leaves: Application to the evaluation of resistance to downy mildew. J. Microbiol. Methods 2013, 95, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Krzyzaniak, Y.; Trouvelot, S.; Negrel, J.; Cluzet, S.; Valls, J.; Richard, T.; Bougaud, A.; Jacquens, L.; Klinguer, A.; Chiltz, A.; et al. A plant extract acts both as a resistance inducer and an oomycide against grapevine downy mildew. Front. Plant Sci. 2018, 9, 1085. [Google Scholar] [CrossRef] [PubMed]






| Experiment 1 | Experiment 2 | Experiment 3 | Mean ÷ Value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | CAS # | Formula | D1 | D3 | D5 | p | D1 | D3 | D5 | p | D1 | D3 | D5 | p | D1 | D3 | D5 |
| α-Farnesene | 21499-64-9 | C15H24 | 0.0025 | <0.001 | ● | ● | ● | ||||||||||
| β-Caryophyllene | 87-44-5 | C15H24 | <0.001 | ● | ● | ||||||||||||
| 2-Oxo-4-phenyl-6-(4-chlorophenyl)-1,2-dihydropyrimidine | 24030-13-5 | C16H11ClN2O | 0.0233 | 0.0338 | ● | ● | ● | ||||||||||
| 4,4’-bi-4H-pyran, 2,2’,6,6’-tetrakis(1,1-dimethylethyl)-4,4’-dimethyl- | 1000399-10-0 | C28H46O2 | 0.0346 | ● | ● | ||||||||||||
| Toluene | 108-88-3 | C7H8 | 0.0430 | ● | |||||||||||||
| 3,4-Dihydroisoquinolin-7-ol, 1-[4-hydroxybenzyl]-6-methoxy- | 47145-46-0 | C17H17NO3 | 0.0018 | ● | ● | ||||||||||||
| Decanal | 112-31-2 | C10H20O | 0.0509 | ● | ● | ● | |||||||||||
| Bastid® | Redeli® | Romeo® | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | CAS # | Formula | D1 | D3 | D5 | D1 | D3 | D5 | D1 | D3 | D5 | Group |
| α-Farnesene | 21499-64-9 | C15H24 | ● | ● | ● | ● | ● | ● | ● | all | ||
| 2-Oxo-4-phenyl-6-(4-chlorophenyl)-1,2-dihydropyrimidine | 24030-13-5 | C16H11ClN2O | ● | ● | ● | ● | ● | ● | ● | all | ||
| Oxime-, methoxy-phenyl- | 1000222-86-6 | C8H9NO2 | ● | ● | ● | ● | ● | ● | all | |||
| 4,4’-bi-4H-pyran, 2,2’,6,6’-tetrakis(1,1-dimethylethyl)-4,4’-dimethyl- | 1000399-10-0 | C28H46O2 | ● | ● | ● | ● | ● | ● | ● | all | ||
| 1,3-benzenedicarboxylic acid, 5-(dimethylamino)- | 1000400-59-0 | C10H11NO4 | ● | ● | ● | ● | ● | ● | ● | all | ||
| β-Caryophyllene | 87-44-5 | C15H24 | ● | ● | ● | ● | ● | ● | all | |||
| Tridecane | 629-50-5 | C13H28 | ● | ● | ● | ● | ● | ● | ● | all | ||
| Decane, 3,8-dimethyl- | 17312-55-9 | C12H26 | ● | ● | ● | ● | ● | ● | ● | all | ||
| Cyclobutanol | 2919-23-5 | C4H8O | ● | ● | ● | ● | ● | ● | ● | all | ||
| Decane, 2,3,8-trimethyl- | 62238-14-6 | C13H28 | ● | ● | ● | ● | ● | ● | ● | Redeli/Romeo | ||
| Decanal | 112-31-2 | C10H20O | ● | ● | ● | ● | ● | Bastid | ||||
| Dodecane | 112-40-3 | C12H26 | ● | ● | ● | ● | ● | Bastid/Romeo | ||||
| Toluene | 108-88-3 | C7H8 | ● | ● | ● | ● | all | |||||
| Carbazole, 2,4,6-trimethyl- | 78787-89-0 | C15H15N | ● | ● | ● | ● | ● | all | ||||
| 4-Hexen-1-ol-acetate | 72237-36-6 | C8H14O2 | ● | ● | ● | ● | ● | ● | all | |||
| Limonene | 5989-27-5 | C10H16 | ● | ● | ● | ● | ● | ● | Redeli/Romeo | |||
| Nonane, 2,2,4,4,6,8,8-heptamethyl- | 4390-04-9 | C16H34 | ● | ● | ● | Bastid | ||||||
| 3,4-Dihydroisoquinolin-7-ol, 1-[4-hydroxybenzyl]-6-methoxy- | 47145-46-0 | C17H17NO3 | ● | ● | ● | ● | Bastid | |||||
| n-Hexadecanoic acid | 112-39-0 | C16H32O2 | ● | ● | ● | ● | Bastid | |||||
| Benzene, (1-ethyldecyl)- | 2400-00-2 | C18H30 | ● | ● | ● | ● | ● | Bastid/Romeo | ||||
| Borane, diethyl(decyloxy)- | 1000152-34-3 | C14H31BO | ● | ● | ● | ● | ● | Bastid/Redeli | ||||
| Undecane, 3,5-dimethyl- | 17312-82-2 | C13H28 | ● | ● | ● | ● | Bastid/Romeo | |||||
| Sulphurous acid, 2-ethylhexyl hexyl ester | 1000309-20-2 | C14H30O3S | ● | ● | ● | ● | Bastid/Romeo | |||||
| Succinic acid, 2,4,6-trichlorophenyl 2-naphthylmethyl ester | 1000390-01-0 | C21H15Cl3O4 | ● | ● | ● | ● | ● | Redeli/Romeo | ||||
| 2,5-Cyclohexadiene, 1,4-diethyl-1,4-dimethyl- | 1000150-21-6 | C12H20 | ● | ● | ● | Bastid | ||||||
| 3-Pentanamine | 616-24-0 | C5H13N | ● | ● | ● | Bastid | ||||||
| Benzene, (1-butyloctyl)- | 2719-63-3 | C18H30 | ● | ● | ● | ● | Bastid/Romeo | |||||
| Pyrimidine-2,4(1H,3H)-dione, 5-amino-6-nitroso- | 1000270-67-7 | C4H4N4O3 | ● | ● | ● | ● | Redeli | |||||
| 3-Ethyl-3-methylheptane | 17302-01-1 | C10H22 | ● | ● | ● | ● | Redeli | |||||
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 84-69-5 | C16H22O4 | ● | ● | ● | ● | Romeo | |||||
| 4-Chlorobenzoic acid, 4-nitrophenyl ester | 1000307-76-2 | C13H8ClNO4 | ● | ● | ● | ● | Redeli/Romeo | |||||
| Benzaldehyde | 78725-46-9 | C7H6O | ● | ● | ● | ● | Redeli/Romeo | |||||
| Methenamine | 100-97-0 | C6H12N4 | ● | ● | ● | ● | Romeo | |||||
| α-Pinene | 80-56-8 | C10H16 | ● | ● | ● | Bastid | ||||||
| Nonanal | 124-19-6 | C9H18O | ● | ● | Bastid | |||||||
| Methyl methacrylate | 80-62-6 | C5H8O2 | ● | ● | ● | Bastid | ||||||
| cis-muurola-4(14),5-diene | 157477-72-0 | C15H24 | ● | ● | Redeli | |||||||
| 3-Benzoyl-2-t-butyl-4-isopropyloxazolidin-5-one | 104057-68-3 | C17H23NO3 | ● | ● | ● | Redeli | ||||||
| Undecane, 2,6-dimethyl- | 17301-23-4 | C13H28 | ● | ● | ● | Redeli | ||||||
| Methyl salicylate | 119-36-8 | C8H8O3 | ● | ● | ● | Romeo | ||||||
| Tetradecane | 629-59-4 | C14H30 | ● | ● | ● | Romeo | ||||||
| Butanoic acid, 2-methyl-, 1,2-dimethylpropyl ester | 84696-83-3 | C10H20O2 | ● | Redeli | ||||||||
| 4-Ethylbenzoic acid, 2-formyl-4,6-dichlorophenyl ester | 1000331-31-6 | C16H12Cl2O3 | ● | ● | Romeo | |||||||
| Product | Manufacturer | Active Component | Doses |
|---|---|---|---|
| Bastid® | Syngenta (Guyancourt, France) | chitooligosaccharide-oligogalacturonic acid | 0.8 mL/L |
| chitosan | Elicityl (Crolles, France) | chitooligosaccharide DP ~13 | 1 g/L |
| OG | Goëmar (Saint-Malo, France) | oligogalacturonic acid DP ~50 | 2.5 g/L |
| Romeo® | BASF (Ecully, France) | yeast cell wall (Saccharomyces cerevisiae LAS117) | 2 g/L |
| Redeli® | Syngenta (Guyancourt, France) | disodium phosphonate | 1 g/L |
| Bion® WG 50 | Syngenta (Guyancourt, France) | acibenzolar-S-methyl | 0.5 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemaitre-Guillier, C.; Dufresne, C.; Chartier, A.; Cluzet, S.; Valls, J.; Jacquens, L.; Douillet, A.; Aveline, N.; Adrian, M.; Daire, X. VOCs Are Relevant Biomarkers of Elicitor-Induced Defences in Grapevine. Molecules 2021, 26, 4258. https://doi.org/10.3390/molecules26144258
Lemaitre-Guillier C, Dufresne C, Chartier A, Cluzet S, Valls J, Jacquens L, Douillet A, Aveline N, Adrian M, Daire X. VOCs Are Relevant Biomarkers of Elicitor-Induced Defences in Grapevine. Molecules. 2021; 26(14):4258. https://doi.org/10.3390/molecules26144258
Chicago/Turabian StyleLemaitre-Guillier, Christelle, Christelle Dufresne, Agnès Chartier, Stéphanie Cluzet, Josep Valls, Lucile Jacquens, Antonin Douillet, Nicolas Aveline, Marielle Adrian, and Xavier Daire. 2021. "VOCs Are Relevant Biomarkers of Elicitor-Induced Defences in Grapevine" Molecules 26, no. 14: 4258. https://doi.org/10.3390/molecules26144258