Roseabol A, a New Peptaibol from the Fungus Clonostachys rosea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of the Clonostachys rosea Metabolites
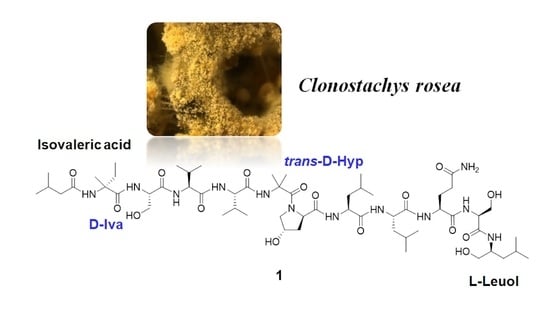
2.2. Structural Characterization of Roseabol A (1): Composition and Amino Acid Sequence
2.3. Assignment of the Relative and Absolute Configuration of Roeseabol A (1)
2.4. Characterization of 13-Oxo-Trans-9,10-Epoxy-11(E)-Octadecenoic Acid (2)
2.5. Assessment of Activity Against Merkel Cell Carcinoma
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Isolation, Culture, and Extraction
3.3. Compound Isolation
3.4. Acid Hydrolysis of Roseabol A (1) and LC-MS Analysis of Marfey’s Derivatives
3.5. Merkel Cell Carcinoma Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Daniel, J.F.S.; Filho, E.R. Peptaibols of Trichoderma. Nat. Prod. Rep. 2007, 24, 1128–1141. [Google Scholar] [CrossRef]
- Bills, G.; Li, Y.; Chen, L.; Yue, Q.; Niu, X.-M.; An, Z. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics. Nat. Prod. Rep. 2014, 31, 1348–1375. [Google Scholar] [CrossRef] [PubMed]
- Dornberger, K.; Ihn, W.; Ritzau, M.; Gräfe, U.; Schlegel, B.; Fleck, W.F. Chrysospermins, new peptaibol antibiotics from Apiocrea chrysosperma Ap101. J. Antibiot. 1995, 48, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Neuhof, T.; Berg, A.; Besl, H.; Schwecke, T.; Dieckmann, R.; von Döhren, H. Peptaibol production by Sepedonium strains parasitizing boletales. Chem. Biodivers. 2007, 4, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Chikanishi, T.; Hasumi, K.; Harada, T.; Kawasaki, N.; Endo, A. Clonostachin, a novel peptaibol that inhibits platelet aggregation. J. Antibiot. 1997, 50, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, C.; Tuttobello, L.; Ricci, M.; Casinovi, C.G.; Radios, L. Ucinostatin D, a novel peptide antibiotic from Paecilomyces Marquandii. J. Antibiot. 1987, 40, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Toniolo, C.; Crisma, M.; Formaggio, F.; Peggion, C.; Epand, R.F. Lipopeptaibols, a novel family of membrane active, antimicrobial peptides. Cell. Mol. Life Sci. 2001, 58, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, G.; Goulard, C.; Prigent, Y.; Bodo, B.; Wróblewski, H.; Rebuffat, S. Sequences and antimycoplasmic properties of longibrachins LGB II and LGB III, two novel 20-residue peptaibols from Trichoderma longibrachiatum. J. Nat. Prod. 2001, 64, 164–170. [Google Scholar] [CrossRef]
- Hosotani, N.; Kumagai, K.; Honda, S.; Ito, A.; Shimatani, T.; Saji, I. SPF-5506-A4, a new peptaibol inhibitor of amyloid-peptide formation produced by Trichoderma sp. J. Antibiot. 2007, 60, 184–190. [Google Scholar] [CrossRef]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current united states incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463. [Google Scholar] [CrossRef]
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I. Merkel cell carcinoma: The biology and treatment of merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 9, 763–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornburg, C.C.; Britt, J.R.; Evans, J.R.; Akee, R.K.; Whitt, J.A.; Trinh, S.K.; Harris, M.J.; Thompson, J.R.; Ewing, T.L.; Shipley, S.M.; et al. NCI program for natural product discovery: A publicly-accessible library of natural product fractions for high-throughput screening. ACS Chem. Biol. 2018, 13, 2484–2497. [Google Scholar] [CrossRef]
- Grkovic, T.; Akee, R.K.; Thornburg, C.C.; Trinh, S.; Britt, J.R.; Harris, M.J.; Evans, J.; Kang, U.; Ensel, S.; Henrich, C.J.; et al. National cancer institute (NCI) program for natural products discovery: Rapid isolation and identification of biologically active natural products from the NCI prefractionated library. ACS Chem. Biol. 2020, 15, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, H.W.; Kleiman, R.; Weisleder, D. Homolytic decomposition of linoleic acid hydroperoxide: Identification of fatty acid products. Lipids 1974, 9, 697–706. [Google Scholar] [CrossRef]
- Liu, D.; Lin, H.; Proksch, P.; Tang, X.; Shao, Z.; Lin, W. Microbacterins A and B, new peptaibols from the deep sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T). Org. Lett. 2015, 17, 1220–1223. [Google Scholar] [CrossRef]
- Ritzau, M.; Heinze, S.; Dornberger, K.; Berg, A.; Fleck, W.; Schlegel, B.; Härtl, A.; Gräfe, U. Ampullosporin, a new peptaibol-type antibiotic from Sepedonium ampullosporum HKI-0053 with neuroleptic activity in mice. J. Antibiot. 1997, 50, 722–728. [Google Scholar] [CrossRef]
- Iijima, M.; Amemiya, M.; Sawa, R.; Kubota, Y.; Kunisada, T.; Momose, I.; Kawada, M.; Shibasaki, M. Acremopeptin, a new peptaibol from Acremonium sp. PF1450. J. Antibiot. 2017, 70, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.-H.; Khalil, Z.; Dewapriya, P.; Salim, A.A.; Lin, H.-W.; Capon, R.J. Trichodermides A–E: New peptaibols isolated from the australian termite nest-derived fungus Trichoderma virens CMB-TN16. J. Nat. Prod. 2018, 81, 976–984. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Mayumi, T.; Oka, H.; Suzuki, M.; Harada, K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Elucidation of limitations of marfey’s method and of its separation mechanism. Anal. Chem. 1997, 69, 3346–3352. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Mayumi, T.; Oka, H.; Suzuki, M.; Harada, K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Combination of marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, J.; Sayre, L.M. Synthesis of six epoxyketooctadecenoic acid (EKODE) isomers, their generation from nonenzymatic oxidation of linoleic acid, and their reactivity with imidazole nucleophiles. J. Org. Chem. 2007, 72, 9471–9480. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.T.; Gould, V.E.; Salwen, H.R.; Herst, C.V.; Le Beau, M.M.; Lee, I.; Bauer, K.; Marder, R.J.; Andersen, R.; Kies, M.S. Establishment and characterization of a neurendocrine skin carcinoma cell line. Lab. Investig. 1987, 56, 302–312. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Adachi, K.; Kanoh, K.; Wisespongp, P.; Nishijima, M.; Shizuri, Y. Clonostachysins A and B, new anti-dinoflagellate cyclic peptides from a marine-derived fungus. J. Antibiot. 2005, 58, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Zhai, M.-M.; Qi, F.-M.; Li, J.; Jiang, C.-X.; Hou, Y.; Shi, Y.-P.; Di, D.-L.; Zhang, J.-W.; Wu, Q.-X. Isolation of Secondary metabolites from the soil-derived fungus Clonostachys rosea YRS-06, a biological control agent, and evaluation of antibiotic activity. J. Agric. Food Chem. 2016, 64, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Bruder, E.D.; Ball, D.L.; Goodfriend, T.L.; Raff, H. An oxidized metabolite of linoleic acid stimulates corticosterone production by rat adrenal cells. Am. J. Physiol. Integr. Comp. Physiol. 2003, 284, R1631–R1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Kern, J.T.; Goodfriend, T.L.; Ball, D.L.; Luesch, H. Activation of the antioxidant response element by specific oxidized metabolites of linoleic acid. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Position | δC, Type | δH, (J in Hz) | Position | δC, Type | δH, (J in Hz) |
|---|---|---|---|---|---|
| Leuol | 3 | 37.0, CH2 (α) | 2.13, m | ||
| 1 | 48.8, CH | 3.77, m | (β) | 1.62, m | |
| 2 | 39.7, CH2 | 1.56, m | 4 | 69.0, CH | 4.25, br s |
| 1.28, m | 5 | 56.4, CH2 (α) | 3.64, br d (11.8) | ||
| 3 | 23.4, CH | 1.45, m | (β) | 3.24, br d (11.8) | |
| 4 | 21.9, CH3 | 0.79, d (6.4) | Aib | ||
| 5 | 20.8, CH3 | 0.77, d (6.4) | 1 | 173.2, C | |
| 6 | 63.8, CH2 | 3.28, m | 2 | 56.1, C | |
| 3.17, m | 3 | 23.1, CH3 | 1.44, s | ||
| NH | 7.28, d (8.9) | 4 | 25.5, CH3 | 1.37, s | |
| Ser1 | NH | 7.90, s | |||
| 1 | 169.3, C | Val1 | |||
| 2 | 55.5, CH | 4.18, dd (9.8, 6.5) | 1 | 172.0, C | |
| 3 | 61.6, CH2 | 3.58, br d (5.6) | 2 | 58.3, CH | 4.13, m |
| NH | 7.62, d (7.9) | 3 | 29.4, CH | 2.15, m | |
| Gln | 4 | 17.8, CH3 | 0.87, d (6.7) | ||
| 1 | 171.0, C | 5 | 19.2, CH3 | 0.94, d (6.7) | |
| 2 | 52.8, CH | 4.14, m | NH | 7.38, d (7.8) | |
| 3 | 27.5, CH2 | 1.90, m | Val2 | ||
| 1.76, m | 1 | 171.5, C | |||
| 4 | 31.5, CH2 | 2.13, m | 2 | 60.4, CH | 3.92, t (6.5) |
| 2.07, m | 3 | 29.1, CH | 2.14, m | ||
| 5 | 173.7, C | 4 | 18.8, CH3 | 0.86, d (6.5) | |
| 2-NH | 7.47, d (6.8) | 5 | 19.0, CH3 | 0.94, d (6.5) | |
| 5-NH2 | 6.77, s | NH | 7.91, d (7.8) | ||
| 7.24, s | Ser2 | ||||
| Leu1 | 1 | 171.4, C | |||
| 1 | 172.2, C | 2 | 57.8, CH | 4.04, m | |
| 2 | 51.2, CH | 4.24, m | 3 | 60.7, CH2 | 3.70, m |
| 3 | 39.2, CH2 | 1.77, m | 3.66, m | ||
| 1.28, m | NH | 8.47, m | |||
| 4 | 24.4, CH | 1.60, m | Iva | ||
| 5 | 22.3, CH3 | 0.87, d (6.6) | 1 | 176.1, C | |
| 6 | 22.2, CH3 | 0.77, d (6.6) | 2 | 58.6, C | |
| NH | 7.35, d (7.8) | 3 | 27.1, CH2 | 1.92, m | |
| Leu2 | 1.69, m | ||||
| 1 | 172.8, C | 4 | 7.6, CH3 | 0.76, t (7.4) | |
| 2 | 52.5, CH | 4.00, m | 5 | 22.1, CH3 | 1.29, s |
| 3 | 38.8, CH2 | 1.86, m | NH | 8.67, s | |
| 1.54, m | Isovaleric acid | ||||
| 4 | 24.0, CH | 1.58, m | 1 | 173.1, C | |
| 5 | 22.8, CH3 | 0.87, d (6.6) | 2 | 44.4, CH2 | 2.11, m |
| 6 | 22.3, CH3 | 0.77, d (6.6) | 2.07, m | ||
| NH | 7.79, d (7.8) | 3 | 25.3, CH | 1.99, m | |
| Hyp | 4 | 22.4, CH3 | 0.79, d (6.7) | ||
| 1 | 173.3, C | 5 | 24.1, CH3 | 0.84, d (6.7) | |
| 2 | 61.3, CH | 4.36, t (8.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-K.; Krumpe, L.R.H.; Smith, E.; Henrich, C.J.; Brownell, I.; Wendt, K.L.; Cichewicz, R.H.; O’Keefe, B.R.; Gustafson, K.R. Roseabol A, a New Peptaibol from the Fungus Clonostachys rosea. Molecules 2021, 26, 3594. https://doi.org/10.3390/molecules26123594
Kim C-K, Krumpe LRH, Smith E, Henrich CJ, Brownell I, Wendt KL, Cichewicz RH, O’Keefe BR, Gustafson KR. Roseabol A, a New Peptaibol from the Fungus Clonostachys rosea. Molecules. 2021; 26(12):3594. https://doi.org/10.3390/molecules26123594
Chicago/Turabian StyleKim, Chang-Kwon, Lauren R. H. Krumpe, Emily Smith, Curtis J. Henrich, Isaac Brownell, Karen L. Wendt, Robert H. Cichewicz, Barry R. O’Keefe, and Kirk R. Gustafson. 2021. "Roseabol A, a New Peptaibol from the Fungus Clonostachys rosea" Molecules 26, no. 12: 3594. https://doi.org/10.3390/molecules26123594