Integration of Mesoporous Bioactive Glass Nanoparticles and Curcumin into PHBV Microspheres as Biocompatible Composite for Drug Delivery Applications
Abstract
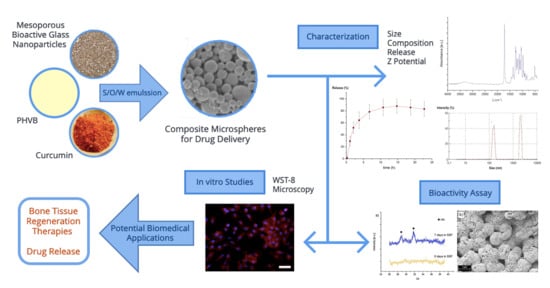
:1. Introduction
2. Results
2.1. Microsphere Size and Zeta Potential
2.2. Surface Morphology of Composite Microspheres
2.3. Composition Analysis
2.4. Curcumin Entrapment Efficiency
2.5. Curcumin Release Kinetics in PBS
2.6. In Vitro Cell Culture Assays
2.7. Bioactivity Assessment
3. Discussion
3.1. Microsphere Size and Z-Potential
3.2. Composite Microspheres as Drug Delivery Systems
3.3. Cytocompatibility of Composite Microspheres
4. Materials and Methods
4.1. Materials
4.2. Synthesis of MBGN
4.3. Composite Microsphere Fabrication
4.4. Curcumin Entrapment Efficiency
4.5. Microsphere Surface Morphology
4.6. Microspheres Size and Zeta Potential Analysis
4.7. Structural Characterization of Composite Microspheres
4.8. Bioactivity Assessment
4.9. Curcumin Release Kinetics
4.10. Cell Culture Assays
4.11. Cell Staining
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zafar, M.S.; Farooq, I.; Awais, M.; Najeeb, S.; Khurshid, Z.; Zohaib, S. Bioactive Surface Coatings for Enhancing Osseointegration of Dental Implants. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–329. [Google Scholar]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.; Goldmann, W.; Boccaccini, A. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: Improving early stage performance of titanium implants. Surf. Coat. Technol. 2020, 381, 125138. [Google Scholar] [CrossRef]
- Doğrul, F.; Bernardo, E.; Boccaccini, A.R.; Galusek, D. Production of SiOC Based Bioactive Glass for Bone-Tissue Applications. In FunGlass School 2019/Part 1; Trencin, Slovaki, 2019; Available online: https://www.funglass.eu/wp-content/uploads/2019/05/FunGlass-School-2019-1_Book-of-abstracts-%C4%8Certov.pdf#page=10 (accessed on 18 May 2021)ISBN 978-80-8075-874-5.
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef]
- Penide, J.; Quintero, F.; del Val, J.; Comesaña, R.; Lusquiños, F.; Riveiro, A. Chapter 10-Bioactive glass nanofibers for tissue engineering. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 329–356. [Google Scholar]
- Nawaz, Q.; Rehman, M.A.U.; Burkovski, A.; Schmidt, J.; Beltrán, A.M.; Shahid, A.; Alber, N.K.; Peukert, W.; Boccaccini, A.R. Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2018, 29, 64. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Bretcanu, O.; Misra, S.K.; Yunos, D.M.; Boccaccini, A.R.; Roy, I.; Kowalczyk, T. Electrospun nanofibrous biodegradable polyester coatings on Bioglass (R)-based glass-ceramics for tissue engineering. Mater. Chem. Phys. 2009, 118, 420–426. [Google Scholar] [CrossRef]
- Reakasame, S.; Trapani, D.; Detsch, R.; Boccaccini, A.R. Cell laden alginate-keratin based composite microcapsules containing bioactive glass for tissue engineering applications. J. Mater. Sci. Mater. Med. 2018, 29, 185. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Boccaccini, A.R.; Knowles, J.C.; Locke, I.C.; Gordge, M.P.; McCormick, A.; Salih, V.; Mordon, N.; Keshavarz, T.; Roy, I.; et al. Fabrication of a novel poly(3-hydroxyoctanoate)/nanoscale bioactive glass composite film with potential as a multifunctional wound dressing. In Proceedings of the 5th International Conference on Times of Polymers Top and Composites, Ischia, Italy, 20–23 June 2010; Damore, A., Acierno, D., Grassia, L., Eds.; Amer Inst Physics: Melville, Australia, 2010; pp. 126–128. [Google Scholar]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Macías-Andrés, V.I.; Li, W.; Aguilar-Reyes, E.A.; Ding, Y.; Roether, J.A.; Harhaus, L. Preparation and characterization of 45S5 bioactive glass-based scaffolds loaded with PHBV microspheres with daidzein release function. J. Biomed. Mater. Res. Part A 2017, 105, 1765–1774. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.C.; Knowles, J.C.; Roy, I.; Boccaccini, A.R. Multi-functional P(3HB) microsphere/45S5 Bioglass (R)-based composite scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 2773–2786. [Google Scholar] [CrossRef]
- Foroughi, M.R.; Hashemi-Beni, B.; Khoroushi, M.; Karbasi, S.; Khademi, A.A. Cytotoxicity assessment of polyhydroxybutyrate/chitosan/nano-bioglass nanofiber scaffolds by stem cells from human exfoliated deciduous teeth stem cells from dental pulp of exfoliated deciduous tooth. Dent. Res. J. 2018, 15, 136–145. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.C.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Monnier, A.; Rombouts, C.; Kouider, D.; About, I.; Fessi, H.; Sheibat-Othman, N. Preparation and characterization of biodegradable polyhydroxybutyrate-co-hydroxyvalerate/polyethylene glycol-based microspheres. Int. J. Pharm. 2016, 513, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.; Meng, D.; Knowles, J.C.; Keshavarz, T.; Boccaccini, A.R.; Roy, I. Controlled Delivery of Gentamicin Using Poly(3-hydroxybutyrate) Microspheres. Int. J. Mol. Sci. 2011, 12, 4294–4314. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Dawn, S.; Samanvitha, K.S.; Kumar, S.S.; Kumar, G.N.; Samrot, A.V. Optimization and characterization of poly[R]hydroxyalkanoate of Pseudomonas aeruginosa SU-1 to utilize in nanoparticle synthesis for curcumin delivery. Biocatal. Agric. Biotechnol. 2017, 12, 292–298. [Google Scholar] [CrossRef]
- Abd El-Hay, A.M.; Naser, A.M.; Badawi, A.; Abd El-Ghaffar, M.A.; Abd El-Wahab, H.; Helal, D.A. Biodegradable polymeric microcapsules for sustained release of riboflavin. Int. J. Biol. Macromol. 2016, 92, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zaloga, J.; Ding, Y.; Liu, Y.; Janko, C.; Pischetsrieder, M.; Alexiou, C.; Boccaccini, A.R. Facile preparation of multifunctional superparamagnetic PHBV microspheres containing SPIONs for biomedical applications. Sci. Rep. 2016, 6, 23140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Rabiela, A.E.; Hernández-Cooper, E.M.; Otero, J.A.; Vergara-Porras, B. Modeling the release of curcumin from microparticles of poly(hydroxybutyrate) [PHB]. Int. J. Biol. Macromol. 2020, 144, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Gopinath, D.; Ahmed, M.; Gomathi, K.; Chitra, K.; Sehgal, P.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sah, H. Simple emulsion technique as an innovative template for preparation of porous, spongelike poly(lactide-co-glycolide) microspheres with pore-closing capability. J. Mater. Sci. 2016, 51, 6257–6274. [Google Scholar] [CrossRef]
- Swornakumari, C.; Meignanalakshmi, S.; Legadevi, R.; Palanisammi, A. Preparation of microspheres using poly-3-hydroxybutyrate biopolymer and its characterization. J. Environ. Biol. 2018, 39, 331–338. [Google Scholar] [CrossRef]
- Zheng, K.; Taccardi, N.; Beltrán, A.M.; Sui, B.; Zhou, T.; Marthala, V.R.R.; Hartmann, M.; Boccaccini, A.R. Timing of calcium nitrate addition affects morphology, dispersity and composition of bioactive glass nanoparticles. RSC Adv. 2016, 6, 95101–95111. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Wu, J.; Li, W.; Dippold, D.; Wan, Y.; Boccaccini, A.R. Incorporation of Cu-Containing Bioactive Glass Nanoparticles in Gelatin-Coated Scaffolds Enhances Bioactivity and Osteogenic Activity. ACS Biomater. Sci. Eng. 2018, 4, 1546–1557. [Google Scholar] [CrossRef]
- Kazemi-Darabadi, S.; Nayebzadeh, R.; Shahbazfar, A.A.; Kazemi-Darabadi, F.; Fathi, E. Curcumin and Nanocurcumin Oral Supplementation Improve Muscle Healing in a Rat Model of Surgical Muscle Laceration. Bull. Emerg. Trauma 2019, 7, 292–299. [Google Scholar] [CrossRef]
- Chidambaram, M.; Krishnasamy, K. Drug-Drug/Drug-Excipient Compatibility Studies on Curcumin using Non-Thermal Methods. Adv. Pharm. Bull. 2014, 4, 309–312. [Google Scholar]
- Athira, G.K.; Jyothi, A.N. Preparation and Characterization of Curcumin Loaded Cassava Starch Nanoparticles Improv. Cell. Absorpt. Int. J. Pharm. Pharm. Sci. 2014, 6, 171–176. [Google Scholar]
- Li, W.; Ding, Y.; Rai, R.; Roether, J.A.; Schubert, D.W.; Boccaccini, A.R. Preparation and characterization of PHBV microsphere/45S5 bioactive glass composite scaffolds with vancomycin releasing function. Mater. Sci. Eng. C 2014, 41, 320–328. [Google Scholar] [CrossRef]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Vu, L.V.; Dung, P.T. Fabrication and vibration characterization of curcumin extracted from turmeric (Curcuma longa) rhizomes of the northern Vietnam. SpringerPlus 2016, 5, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panith, N.; Assavanig, A.; Lertsiri, S.; Bergkvist, M.; Surarit, R.; Niamsiri, N. Development of tunable biodegradable polyhydroxyalkanoates microspheres for controlled delivery of tetracycline for treating periodontal disease. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.; De Melo, N.F.S.; Porto, R.M.; Feitosa, L.O.; Tonello, P.S.; Filho, N.L.D.; Rosa, A.H.; Lima, R.; Fraceto, L. Controlled release system for ametryn using polymer microspheres: Preparation, characterization and release kinetics in water. J. Hazard. Mater. 2011, 186, 1645–1651. [Google Scholar] [CrossRef] [Green Version]
- Conoscenti, G.; Pavia, F.C.; Ciraldo, F.E.; Liverani, L.; Brucato, V.; La Carrubba, V.; Boccaccini, A.R. In vitro degradation and bioactivity of composite poly-l-lactic (PLLA)/bioactive glass (BG) scaffolds: Comparison of 45S5 and 1393BG compositions. J. Mater. Sci. 2018, 53, 2362–2374. [Google Scholar] [CrossRef]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.-P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, L.; Thompson, D.M.; Montoya, L.D. Effect of particle size on in vitro cytotoxicity of titania and alumina nanoparticles. J. Exp. Nanosci. 2014, 9, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, S.; Yan, Y.; Su, J.; Wang, D.; Zhao, J.; Wang, S.; Zhang, X. Absorbable nanocomposites composed of mesoporous bioglass nanoparticles and polyelectrolyte complexes for surgical hemorrhage control. Mater. Sci. Eng. C 2020, 109, 110556. [Google Scholar] [CrossRef]
- Masood, F.; Chen, P.; Yasin, T.; Hasan, F.; Ahmad, B.; Hameed, A. Synthesis of poly-(3-hydroxybutyrate-co-12 mol % 3-hydroxyvalerate) by Bacillus cereus FB11: Its characterization and application as a drug carrier. J. Mater. Sci. Mater. Med. 2013, 24, 1927–1937. [Google Scholar] [CrossRef]
- Singh, P.K.; Wani, K.; Kaul-Ghanekar, R.; Prabhune, A.; Ogale, S. From micron to nano-curcumin by sophorolipid co-processing: Highly enhanced bioavailability, fluorescence, and anti-cancer efficacy. RSC Adv. 2014, 4, 60334–60341. [Google Scholar] [CrossRef]
- Zidan, A.S.; Rahman, Z.; Khan, M.A. Product and process understanding of a novel pediatric anti-HIV tenofovir niosomes with a high-pressure homogenizer. Eur. J. Pharm. Sci. 2011, 44, 93–102. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Rabiela, A.E.; Leal-Egaña, A.; Nawaz, Q.; Boccaccini, A.R. Integration of Mesoporous Bioactive Glass Nanoparticles and Curcumin into PHBV Microspheres as Biocompatible Composite for Drug Delivery Applications. Molecules 2021, 26, 3177. https://doi.org/10.3390/molecules26113177
Aguilar-Rabiela AE, Leal-Egaña A, Nawaz Q, Boccaccini AR. Integration of Mesoporous Bioactive Glass Nanoparticles and Curcumin into PHBV Microspheres as Biocompatible Composite for Drug Delivery Applications. Molecules. 2021; 26(11):3177. https://doi.org/10.3390/molecules26113177
Chicago/Turabian StyleAguilar-Rabiela, Arturo E., Aldo Leal-Egaña, Qaisar Nawaz, and Aldo R. Boccaccini. 2021. "Integration of Mesoporous Bioactive Glass Nanoparticles and Curcumin into PHBV Microspheres as Biocompatible Composite for Drug Delivery Applications" Molecules 26, no. 11: 3177. https://doi.org/10.3390/molecules26113177