Novel Tdp1 Inhibitors Based on Adamantane Connected with Monoterpene Moieties via Heterocyclic Fragments
Abstract
:1. Introduction
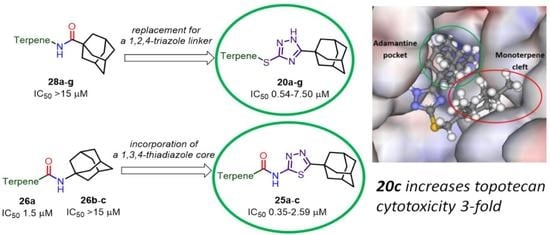
2. Results and Discussion
2.1. Chemistry
2.2. Biological Assays
2.3. Molecular Modeling
2.4. Chemical Space
3. Materials and Methods
3.1. Chemistry
- Synthesis of 2-(adamantane-1-carbonyl)hydrazine-1-carbothioamide 15
- Synthesis of 5-(adamantan-1-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 16
- General procedure for obtaining 1,2,4-triazole derivatives 20a–20g
- 5-(Adamantan-1-yl)-3-((3,7-dimethyloctyl)thio)-1H-1,2,4-triazole 20a

- 5-(Adamantan-1-yl)-3-(((S)-3,7-dimethyloct-6-en-1-yl)thio)-1H-1,2,4-triazole 20b

- 5-(Adamantan-1-yl)-3-(((E)-3,7-dimethylocta-2,6-dien-1-yl)thio)-1H-1,2,4-triazole 20c

- 5-(Adamantan-1-yl)-3-(((Z)-3,7-dimethylocta-2,6-dien-1-yl)thio)-1H-1,2,4-triazole 20d

- 5-(Adamantan-1-yl)-3-((((S)-4-(prop-1-en-2-yl)cyclohex-1-en-1-yl)methyl)thio)-1H-1,2,4-triazole 20e

- 5-(Adamantan-1-yl)-3-((((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)thio)-1H-1,2,4-triazole 20f

- 5-(Adamantan-1-yl)-3-((2-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)ethyl)thio)-1H-1,2,4-triazole 20g

- General procedure for synthesis of bromides
- Synthesis of 5-(adamantan-1-yl)-1,3,4-thiadiazol-2-amine 17
- Synthesis of 3,7-dimethyloctanoic acid 23a
- Synthesis of (-)-myrtenic acid 23c
- Synthesis of (S)-4-(prop-1-en-2-yl)cyclohex-1-ene-1-carboxylic acid 23d
- General procedure for acid chloride synthesis
- General procedure for obtaining 25a–d and 26a
- N-(5-(Adamantan-1-yl)-1,3,4-thiadiazol-2-yl)-3,7-dimethyloctanamide 25a

- N-(5-(Adamantan-1-yl)-1,3,4-thiadiazol-2-yl)-3,7-dimethyloct-6-enamide 25b

- (1R,5S)-N-(5-(Adamantan-1-yl)-1,3,4-thiadiazol-2-yl)-6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-carboxamide 25c

- (S)-N-(5-(adamantan-1-yl)-1,3,4-thiadiazol-2-yl)-4-(prop-1-en-2-yl)cyclohex-1-ene-1-carboxamide 25d

- N-(Adamantan-1-yl)-3,7-dimethyloctanamide 26a

3.2. Biology
3.2.1. Detection of Tdp1 Activity
3.2.2. Cytotoxicity Assays
3.3. Molecular Modeling and Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Tdp1 | Tyrosyl-DNA phosphodiesterase 1 |
| DMSO | Dimethyl sulfoxide |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Morham, S.G.; Kluckman, K.D.; Voulomanos, N.; Smithies, O. Targeted disruption of the mouse topoisomerase I gene by camptothecin selection. Mol. Cell. Biol. 1996, 16, 6804–6809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, J.A. DNA topoisomerases as anticancer drug targets: From the laboratory to the clinic. Curr. Med. Chem. Anticancer Agents 2001, 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wu, Q.; Luan, S.; Yin, Z.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. A comprehensive review of topoisomerase inhibitors as anticancer agents in the past decade. Eur. J. Med. Chem. 2019, 171, 129–168. [Google Scholar] [CrossRef]
- Pommier, Y.; Barcelo, J.M.; Rao, V.A.; Sordet, O.; Jobson, A.G.; Thibaut, L.; Miao, Z.H.; Seiler, J.A.; Zhang, H.; Marchand, C.; et al. Repair of topoisomerase I-mediated DNA damage. Prog. Nucleic Acid Res. Mol. Biol. 2006, 81, 179–229. [Google Scholar] [CrossRef] [Green Version]
- Murai, J.; Huang, S.Y.N.; Das, B.B.; Dexheimer, T.S.; Takeda, S.; Pommier, Y. Tyrosyl-DNA phosphodiesterase 1 (TDP1) repairs DNA damage induced by topoisomerases I and II and base alkylation in vertebrate cells. J. Biol. Chem. 2012, 287, 12848–12857. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.; Antony, S.; Kohn, K.W.; Cushman, M.; Ioanoviciu, A.; Staker, B.L.; Burgin, A.B.; Stewart, L.; Pommier, Y. A novel norindenoisoquinoline structure reveals a common interfacial inhibitor paradigm for ternary trapping of the topoisomerase I-DNA covalent complex. Mol. Cancer Ther. 2006, 5, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.W.; Burgin, A.B.; Huizenga, B.N.; Robertson, C.A.; Yao, K.C.; Nash, H.A. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc. Natl. Acad. Sci. USA 1996, 93, 11534–11539. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhou, S.; Begum, S.; Sidransky, D.; Westra, W.H.; Brock, M.; Califano, J.A. Increased expression and activity of repair genes Tdp1 and XPF in non-small cell lung cancer. Lung Cancer 2007, 55, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Rodriguez-Silva, M.; de la Rocha, A.M.A.; Wolf, A.L.; Lai, Y.; Liu, Y.; Reinhold, W.C.; Pommier, Y.; Chambers, J.W.; Tse-Dinh, Y.C. Tyrosyl-DNA phosphodiesterase 1 and topoisomerase I activities as predictive indicators for glioblastoma susceptibility to genotoxic agents. Cancers 2019, 11, 1416. [Google Scholar] [CrossRef] [Green Version]
- El-Khamisy, S.F.; Katyal, S.; Patel, P.; Ju, L.; McKinnon, P.J.; Caldecott, K.W. Synergistic decrease of DNA single-strand break repair rates in mouse neural cells lacking both Tdp1 and aprataxin. DNA Repair 2009, 8, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Katyal, S.; El-Khamisy, S.F.; Russell, H.R.; Li, Y.; Ju, L.; Caldecott, K.W.; McKinnon, P.J. Tdp1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007, 26, 4720–4731. [Google Scholar] [CrossRef] [Green Version]
- Hirano, R.; Interthal, H.; Huang, C.; Nakamura, T.; Deguchi, K.; Choi, K.; Bhattacharjee, M.B.; Arimura, K.; Umehara, F.; Izumo, S.; et al. Spinocerebellar ataxia with axonal neuropathy: Consequence of a Tdp1 recessive neomorphic mutation? EMBO J. 2007, 26, 4732–4743. [Google Scholar] [CrossRef] [Green Version]
- Brettrager, E.J.; van Waardenburg, R.C.A.M. Targeting tyrosyl-DNA phosphodiesterase I to enhance toxicity of phosphodiester linked DNA-adducts. Cancer Drug Resist. 2019, 2, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Mozhaitsev, E.; Suslov, E.; Demidova, Y.; Korchagina, D.; Volcho, K.; Zakharenko, A.; Vasil’eva, I.; Kupryushkin, M.; Chepanova, A.; Ayine-Tora, D.M.; et al. The development of tyrosyl-DNA phosphodyesterase 1 (Tdp1) inhibitors based on the amines combining aromatic/heteroaromatic and monoterpenoid moieties. Lett. Drug Des. Discov. 2018, 16, 597–605. [Google Scholar] [CrossRef]
- Khomenko, T.; Zakharenko, A.; Odarchenko, T.; Arabshahi, H.J.; Sannikova, V.; Zakharova, O.; Korchagina, D.; Reynisson, J.; Volcho, K.; Salakhutdinov, N.; et al. New inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1) combining 7-hydroxycoumarin and monoterpenoid moieties. Bioorg. Med. Chem. 2016, 24, 5573–5581. [Google Scholar] [CrossRef]
- Zakharenko, A.L.; Ponomarev, K.U.; Suslov, E.V.; Korchagina, D.V.; Volcho, K.P.; Vasil’eva, I.A.; Salakhutdinov, N.F.; Lavrik, O.I. Inhibitory properties of nitrogen-containing adamantane derivatives with monoterpenoid fragments against tyrosyl-DNA phosphodiesterase 1. Russ. J. Bioorg. Chem. 2015, 41, 657–662. [Google Scholar] [CrossRef]
- Khomenko, T.M.; Zakharenko, A.L.; Chepanova, A.A.; Ilina, E.S.; Zakharova, O.D.; Kaledin, V.I.; Nikolin, V.P.; Popova, N.A.; Korchagina, D.V.; Reynisson, J.; et al. Promising new inhibitors of tyrosyl-DNA phosphodiesterase I (Tdp 1) combining 4- arylcoumarin and monoterpenoid moieties as components of complex antitumor therapy. Int. J. Mol. Sci. 2020, 21, 126. [Google Scholar] [CrossRef] [Green Version]
- Chepanova, A.A.; Li-Zhulanov, N.S.; Sukhikh, A.S.; Zafar, A.; Reynisson, J.; Zakharenko, A.L.; Zakharova, O.D.; Korchagina, D.V.; Volcho, K.P.; Salakhutdinov, N.F.; et al. Effective inhibitors of tyrosyl-DNA phosphodiesterase 1 based on monoterpenoids as potential agents for antitumor therapy. Russ. J. Bioorg. Chem. 2019, 45, 647–655. [Google Scholar] [CrossRef]
- Il’ina, I.V.; Dyrkheeva, N.S.; Zakharenko, A.L.; Sidorenko, A.Y.; Li-Zhulanov, N.S.; Korchagina, D.V.; Chand, R.; Ayine-Tora, D.M.; Chepanova, A.A.; Zakharova, O.D.; et al. Design, synthesis and biological investigation of novel classes of 3-carene-derived potent inhibitors of Tdp1. Molecules 2020, 25, 3496. [Google Scholar] [CrossRef]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [Green Version]
- Kovaleva, K.; Oleshko, O.; Mamontova, E.; Yarovaya, O.; Zakharova, O.; Zakharenko, A.; Kononova, A.; Dyrkheeva, N.; Cheresiz, S.; Pokrovsky, A.; et al. Dehydroabietylamine ureas and thioureas as tyrosyl-DNA phosphodiesterase 1 inhibitors that enhance the antitumor effect of temozolomide on glioblastoma cells. J. Nat. Prod. 2019, 82, 2443–2450. [Google Scholar] [CrossRef]
- Ponomarev, K.Y.; Suslov, E.V.; Zakharenko, A.L.; Zakharova, O.D.; Rogachev, A.D.; Korchagina, D.V.; Zafar, A.; Reynisson, J.; Nefedov, A.A.; Volcho, K.P.; et al. Aminoadamantanes containing monoterpene-derived fragments as potent tyrosyl-DNA phosphodiesterase 1 inhibitors. Bioorg. Chem. 2018, 76, 392–399. [Google Scholar] [CrossRef]
- Chepanova, A.A.; Mozhaitsev, E.S.; Munkuev, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Zakharenko, A.L.; Patel, J.; Ayine-Tora, D.M.; Reynisson, J.; et al. The development of Tyrosyl-DNA phosphodiesterase 1 inhibitors. Combination of monoterpene and adamantine moieties via amide or thioamide bridges. Appl. Sci. 2019, 9, 2767. [Google Scholar] [CrossRef] [Green Version]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef]
- Luks, A.M.; McIntosh, S.E.; Grissom, C.K.; Auerbach, P.S.; Rodway, G.W.; Schoene, R.B.; Zafren, K.; Hackett, P.H. Wilderness medical society consensus guidelines for the prevention and treatment of acute altitude illness. Wilderness Environ. Med. 2010, 21, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Luzina, O.; Filimonov, A.; Zakharenko, A.; Chepanova, A.; Zakharova, O.; Ilina, E.; Dyrkheeva, N.; Likhatskaya, G.; Salakhutdinov, N.; Lavrik, O. Usnic acid conjugates with monoterpenoids as potent tyrosyl-DNA phosphodiesterase 1 inhibitors. J. Nat. Prod. 2020, 83, 2320–2329. [Google Scholar] [CrossRef]
- Li-Zhulanov, N.S.; Zakharenko, A.L.; Chepanova, A.A.; Patel, J.; Zafar, A.; Volcho, K.P.; Salakhutdinov, N.F.; Reynisson, J.; Leung, I.K.H.; Lavrik, O.I. A novel class of tyrosyl-DNA phosphodiesterase 1 inhibitors that contains the octahydro-2H-chromen-4-ol scaffold. Molecules 2018, 23, 2468. [Google Scholar] [CrossRef] [Green Version]
- Milošev, M.Z.; Jakovljević, K.; Joksović, M.D.; Stanojković, T.; Matić, I.Z.; Perović, M.; Tešić, V.; Kanazir, S.; Mladenović, M.; Rodić, M.V.; et al. Mannich bases of 1,2,4-triazole-3-thione containing adamantane moiety: Synthesis, preliminary anticancer evaluation, and molecular modeling studies. Chem. Biol. Drug Des. 2017, 89, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Fesatidou, M.; Zagaliotis, P.; Camoutsis, C.; Petrou, A.; Eleftheriou, P.; Tratrat, C.; Haroun, M.; Geronikaki, A.; Ciric, A.; Sokovic, M. 5-Adamantan thiadiazole-based thiazolidinones as antimicrobial agents. Design, synthesis, molecular docking and evaluation. Bioorg. Med. Chem. 2018, 26, 4664–4676. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Cooke, N.G.; DeHoff, B.; Sakito, Y. The total synthesis of (+)-ionomycin. J. Am. Chem. Soc. 1990, 112, 5276–5290. [Google Scholar] [CrossRef]
- Godeau, J.; Fontaine-Vive, F.; Antoniotti, S.; Duñach, E. Experimental and theoretical studies on the bismuth-triflate-catalysed cycloisomerisation of 1,6,10-trienes and aryl polyenes. Chem. Eur. J. 2012, 18, 16815–16822. [Google Scholar] [CrossRef] [PubMed]
- Akgun, B.; Hall, D.G. Fast and tight boronate formation for click bioorthogonal conjugation. Angewandte Chemie Int. Ed. 2016, 55, 3909–3913. [Google Scholar] [CrossRef]
- Il’Ina, I.V.; Volcho, K.P.; Korchagina, D.V.; Salakhutdinov, N.F. The convenient way for obtaining geranial by acid-catalyzed kinetic resolution of citral. Helv. Chim. Acta 2016, 99, 373–377. [Google Scholar] [CrossRef]
- Jocelyn, P.C.; Polgar, N. 26. Methyl-substituted αβ-unsaturated acids. Part I. J. Chem. Soc. 1953, 1, 132–137. [Google Scholar] [CrossRef]
- Lin, G.-S.; Duan, W.-G.; Yang, L.-X.; Huang, M.; Lei, F.-H. Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1,2,4-triazole-thioethers. Molecules 2017, 22, 193. [Google Scholar] [CrossRef]
- Khojasteh, S.C.; Oishi, S.; Nelson, S.D. Metabolism and toxicity of menthofuran in rat liver slices and in rats. Chem. Res. Toxicol. 2010, 23, 1824–1832. [Google Scholar] [CrossRef] [Green Version]
- Waghmare, A.A.; Hindupur, R.M.; Pati, H.N. Propylphosphonic anhydride (T3P®): An expedient reagent for organic synthesis. Rev. J. Chem. 2014, 4, 53–131. [Google Scholar] [CrossRef]
- Suslov, E.V.; Mozhaytsev, E.S.; Korchagina, D.V.; Bormotov, N.I.; Yarovaya, O.I.; Volcho, K.P.; Serova, O.A.; Agafonov, A.P.; Maksyutov, R.A.; Shishkina, L.N.; et al. New chemical agents based on adamantane-monoterpene conjugates against orthopoxvirus infections. RSC Med. Chem. 2020, 11, 1185–1195. [Google Scholar] [CrossRef]
- Zakharenko, A.; Khomenko, T.; Zhukova, S.; Koval, O.; Zakharova, O.; Anarbaev, R.; Lebedeva, N.; Korchagina, D.; Komarova, N.; Vasiliev, V.; et al. Synthesis and biological evaluation of novel tyrosyl-DNA phosphodiesterase 1 inhibitors with a benzopentathiepine moiety. Bioorg. Med. Chem. 2015, 23, 2044–2052. [Google Scholar] [CrossRef]
- Antony, S.; Marchand, C.; Stephen, A.G.; Thibaut, L.; Agama, K.K.; Fisher, R.J.; Pommier, Y. Novel high-throughput electrochemiluminescent assay for identification of human tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors and characterization of furamidine (NSC 305831) as an inhibitor of Tdp1. Nucleic Acids Res. 2007, 35, 4474–4484. [Google Scholar] [CrossRef] [Green Version]
- Lountos, G.T.; Zhao, X.Z.; Kiselev, E.; Tropea, J.E.; Needle, D.; Pommier, Y.; Burke, T.R.; Waugh, D.S. Identification of a ligand binding hot spot and structural motifs replicating aspects of tyrosyl-DNA phosphodiesterase I (Tdp1) phosphoryl recognition by crystallographic fragment cocktail screening. Nucleic Acids Res. 2019, 47, 10134–10150. [Google Scholar] [CrossRef] [Green Version]
- Filimonov, A.S.; Chepanova, A.A.; Luzina, O.A.; Zakharenko, A.L.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Kuprushkin, M.S.; Kolotaev, A.V.; Khachatryan, D.S.; et al. New hydrazinothiazole derivatives of usnic acid as potent Tdp1 inhibitors. Molecules 2019, 24, 3711. [Google Scholar] [CrossRef] [Green Version]
- Zafar, A.; Reynisson, J. Hydration free energy as a molecular descriptor in drug design: A feasibility study. Mol. Inform. 2016, 35, 207–214. [Google Scholar] [CrossRef]
- Salomatina, O.V.; Popadyuk, I.I.; Zakharenko, A.L.; Zakharova, O.D.; Chepanova, A.A.; Dyrkheeva, N.S.; Komarova, N.I.; Reynisson, J.; Anarbaev, R.O.; Salakhutdinov, N.F.; et al. Deoxycholic acid as a molecular scaffold for tyrosyl-DNA phosphodiesterase 1 inhibition: A synthesis, structure—Activity relationship and molecular modeling study. Steroids 2021, 165, 108771. [Google Scholar] [CrossRef]
- Zhu, F.; Logan, G.; Reynisson, J. Wine compounds as a source for HTS screening collections. A feasibility study. Mol. Inform. 2012, 31, 847–855. [Google Scholar] [CrossRef]
- Eurtivong, C.; Reynisson, J. The development of a weighted index to optimise compound libraries for high throughput screening. Mol. Inform. 2019, 38. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tamura, T.; Yoshimoto, S.; Kawakami, T.; Masuyama, A. 4-Methyltetrahydropyran (4-MeTHP): Application as an organic reaction solvent. Chem. Asian J. 2019, 14, 3921–3937. [Google Scholar] [CrossRef] [Green Version]
- Janßen, M.A.; Thiele, C.M. Poly-γ-S-perillyl-l-glutamate and poly-γ-S-perillyl-d-glutamate: Diastereomeric alignment media used for the investigation of the alignment process. Chem. Eur. J. 2020, 26, 7831–7839. [Google Scholar] [CrossRef]
- Liu, H.X.; Tan, H.B.; He, M.T.; Li, L.; Wang, Y.H.; Long, C.L. Isolation and synthesis of two hydroxychavicol heterodimers from Piper nudibaccatum. Tetrahedron 2015, 71, 2369–2375. [Google Scholar] [CrossRef]
- Ottenbacher, R.V.; Samsonenko, D.G.; Talsi, E.P.; Bryliakov, K.P. Highly efficient, regioselective, and stereospecific oxidation of aliphatic C-H groups with H2O2, catalyzed by aminopyridine manganese complexes. Org. Lett. 2012, 14, 4310–4313. [Google Scholar] [CrossRef] [PubMed]
- Szczerbowski, D.; Schulz, S.; Zarbin, P.H.G. Total synthesis of four stereoisomers of methyl 4,8,12-trimethylpentadecanoate, a major component of the sex pheromone of the stink bug: Edessa meditabunda. Org. Biomol. Chem. 2020, 18, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The protein data bank. Acta Crystallogr. Sect. D 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Scigress Ultra, V.F. J 2.6. (EU 3.1.7); Fujitsu Limited: Tokyo, Japan, 2008–2016. [Google Scholar]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1and V2Torsional terms1,2. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Goto, H.; Osawa, E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 2 1993, 2, 187–198. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 11, 727–748. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aided Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved Protein-Ligand Docking using GOLD. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced protein-ligand docking with plants. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef]
- Mooij, W.T.M.; Verdonk, M.L. General and targeted statistical potentials for protein-ligand interactions. Proteins Struct. Funct. Genet. 2005, 61, 272–287. [Google Scholar] [CrossRef]
- QikProp; Version 6.2; Schrödinger: New York, NY, USA, 2009.
- Ioakimidis, L.; Thoukydidis, L.; Mirza, A.; Naeem, S.; Reynisson, J. Benchmarking the reliability of QikProp. Correlation between experimental and predicted values. QSAR Comb. Sci. 2008, 27, 445–456. [Google Scholar] [CrossRef]









| Structure | IC50, μM | CC50, μM HeLa | CC50, μM HCT-116 | CC50, μM SW837 | |
|---|---|---|---|---|---|
1,2,4-Triazole derivatives | |||||
| 20a |  | 0.54 ± 0.09 | 85 ± 23 | 27 ± 6 | 52 ± 6 |
| 20b |  | 1.5 ± 0.3 | >100 | >100 | 41 ± 4 |
| 20c |  | 5.3 ± 1.7 | 64 ± 6 | 16 ± 7 | 41 ± 6 |
| 20d |  | 5.6 ± 0.6 | 31± 11 | 20 ± 9 | 17 ± 5 |
| 20e |  | 6.2 ± 2.2 | 24 ± 16 | 15 ± 5 | 40 ± 4 |
| 20f |  | 7.5 ± 1.8 | 15 ± 2 | 54 ± 5 | 46 ± 6 |
| 20g |  | 0.57 ± 0.14 | 74 ± 17 | 34 ± 6 | 40 ± 4 |
1,3,4-Thiadiazole derivatives | |||||
| 25a |  | 0.35 ± 0.05 | >100 | >100 | >100 |
| 25b |  | 2.59 ± 0.48 | >100 | 66 ± 6 | 83 ± 8 |
| 25c |  | 0.45 ± 0.09 | >100 | >100 | >100 |
| Compound | HeLa—CC50, μM | HCT116—CC50, nM |
|---|---|---|
| Topotecan | 6.0 | 715.0 |
| 20a | 4.5 | 650.0 (20) |
| 20b | ND * | 575.3 (20) |
| 20c | 1.9 | 460.0 (15) |
| 20d | 5.4 | 689.0 (15) |
| 20e | ND | 392.0 (15) |
| 20f | 2.8 | 715.0 (25) |
| 20g | 2.6 | 235.8 (20) |
| 25a | 4.9 | 455.7 (45) |
| 25b | 2.9 | 611.9 (20) |
| 25c | 3.6 | 486.0 (25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munkuev, A.A.; Mozhaitsev, E.S.; Chepanova, A.A.; Suslov, E.V.; Korchagina, D.V.; Zakharova, O.D.; Ilina, E.S.; Dyrkheeva, N.S.; Zakharenko, A.L.; Reynisson, J.; et al. Novel Tdp1 Inhibitors Based on Adamantane Connected with Monoterpene Moieties via Heterocyclic Fragments. Molecules 2021, 26, 3128. https://doi.org/10.3390/molecules26113128
Munkuev AA, Mozhaitsev ES, Chepanova AA, Suslov EV, Korchagina DV, Zakharova OD, Ilina ES, Dyrkheeva NS, Zakharenko AL, Reynisson J, et al. Novel Tdp1 Inhibitors Based on Adamantane Connected with Monoterpene Moieties via Heterocyclic Fragments. Molecules. 2021; 26(11):3128. https://doi.org/10.3390/molecules26113128
Chicago/Turabian StyleMunkuev, Aldar A., Evgenii S. Mozhaitsev, Arina A. Chepanova, Evgeniy V. Suslov, Dina V. Korchagina, Olga D. Zakharova, Ekaterina S. Ilina, Nadezhda S. Dyrkheeva, Alexandra L. Zakharenko, Jóhannes Reynisson, and et al. 2021. "Novel Tdp1 Inhibitors Based on Adamantane Connected with Monoterpene Moieties via Heterocyclic Fragments" Molecules 26, no. 11: 3128. https://doi.org/10.3390/molecules26113128