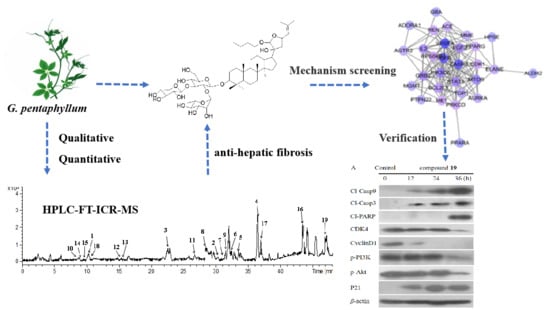
Activity Components from Gynostemma pentaphyllum for Preventing Hepatic Fibrosis and of Its Molecular Targets by Network Pharmacology Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Studies of Gypenosides Extracted from G. pentaphyllum
2.2. Specific Anti-Proliferative Effect of Individual Gypenosides on t-HSC/Cl-6 Cells
2.3. Network Pharmacology Analysis
2.3.1. Screening of Common Targets and Constructing of “Protein–Protein Interaction” (PPI) Networks between Gypenoside and Liver Fibrosis
2.3.2. KEGG Pathways Analysis and GO Enrichment
2.4. The Anti-Hepatic Fibrosis Mechanism of Compound 19
3. Materials and Methods
3.1. Materials and Regents
3.2. Extraction Procedures
3.3. HPLC-FT-ICR MS Analysis
3.4. Simultaneous Determination of Individual Gypenosides
3.5. Cell Proliferation Assays
3.6. Network Pharmacology
3.6.1. Screening of Common Targets of Compound 19 and Liver Fibrosis
3.6.2. The Enrichment Analysis of Gene Ontology (GO) and KEGG Pathway
3.6.3. Construction of “Component–Target–Pathway–Disease” Network
3.7. Cell Cycle Test
3.8. Cell Apoptosis Analysis
3.9. Western Blotting Test
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, C.; Zhang, H.; Bai, R. Advances in ultrasound-targeted microbubble-mediated gene therapy for liver fibrosis. Acta Pharm. Sin. B 2017, 7, 447–452. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Wong, N.K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.F.; Liu, L.H.; Zu, M.L.; Ding, X.F.; Cui, W.Y.; Chang, T.; Piao, X.L. The inhibitory effect of gypenoside stereoisomers, gypenoside L and gypenoside LI, isolated from Gynostemma pentaphyllum on the growth of human lung cancer A549 cells. J. Ethnopharmacol. 2018, 219, 161–172. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.L.; Zhou, P.P.; Meng, X.H.; Shi, Y.P. Further New Gypenosides from Jiaogulan (Gynostemma pentaphyllum). J. Agric. Food Chem. 2017, 65. [Google Scholar] [CrossRef]
- Wan, Z.H.; Zhao, Q. Gypenoside inhibits interleukin-1β-induced inflammatory response in human osteoarthritis chondrocytes. J. Biochem. Mol. Toxicol. 2017. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lee, M.L.; Chan, B.D.; Ma, W.S.; Tai, C.S. Gynostemma pentaphyllum saponins attenuate inflammation in vitro and in vivo by inhibition of NF-κB and STAT3 signaling. Oncotarget 2017, 8, 87401–87414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schild, L.; Cotte, T.; Keilhoff, G.; Brodemann, R. Preconditioning of brain slices against hypoxia induced injury by a Gynostemma pentaphyllum extract--stimulation of anti-oxidative enzyme expression. Phytomedicine 2012, 19, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Du, Y.; Fan, Q.; Tang, C.Y.; He, J.F. Gypenosides might have neuroprotective and immunomodulatory effects on optic neuritis. Med. Hypotheses 2014, 82, 636–638. [Google Scholar] [CrossRef]
- Hung, T.M.; Cao, V.T.; Cuong, T.D.; Hung, N.P.; Kwack, S.J.; Huh, J.I.; Min, B.S.; Choi, J.S.; Lee, H.K.; Bae, K. Dammarane-Type Glycosides from Gynostemma pentaphyllum and Their Effects on IL-4-Induced Eotaxin Expression in Human Bronchial Epithelial Cells. J. Nat. Prod. 2010, 73, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Brar, M.S.; Leung, F.C.C.; Hsiao, W.L.W. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in Apc(Min/+) mice. Oncotarget 2016, 7, 31226–31242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Zhao, M.; Qi, X.; Liu, Y.; Li, N.; Liu, Z.; Bian, Y. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch. Pharm. Res. 2016, 39, 221–230. [Google Scholar] [CrossRef]
- Yassin, K.; Huyen, V.T.; Hoa, K.N.; Ostenson, C.G. Herbal extract of gynostemma pentaphyllum decreases hepatic glucose output in type 2 diabetic goto-kakizaki rats. Int. J. Biomed. Sci. IJBS 2011, 7, 131–136. [Google Scholar] [CrossRef]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Ky, P.T.; Hoa, N.K.; Ostenson, C.G. Antidiabetic Effects of Add-On Gynostemma pentaphyllum Extract Therapy with Sulfonylureas in Type 2 Diabetic Patients. Evid. Based Complement. Altern. Med. 2012, 2012, 452313. [Google Scholar] [CrossRef] [Green Version]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Ostenson, C.G. Gynostemma pentaphyllum Tea Improves Insulin Sensitivity in Type 2 Diabetic Patients. J. Nutr. Metab. 2013, 2013, 765383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Zhang, H.; Luo, Y.; Zhang, J.; Wang, M.; Liao, P.; Cao, L.; Guo, P.; Sun, G.; Sun, X. Gypenoside XVII Prevents Atherosclerosis by Attenuating Endothelial Apoptosis and Oxidative Stress: Insight into the ERalpha-Mediated PI3K/Akt Pathway. Int. J. Mol. Sci. 2017, 18, 77. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Ming, Y.; Wan, Q.; Ye, S.; Xie, S.; Zhu, Y.; Wang, Y.; Zhong, Z.; Li, L.; Ye, Q. Gypenoside attenuates hepatic ischemia/reperfusion injury in mice via anti-oxidative and anti-apoptotic bioactivities. Exp. Ther. Med. 2014, 7, 1388–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.; Wang, X.; Zhang, H.; Zhang, X.; Zhao, Y. New dammarane-type triterpene saponins from Gynostemma pentaphyllum and their anti-hepatic fibrosis activities in vitro. J. Funct. Foods 2018, 45, 10–14. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Available online: http://www.nhc.gov.cn/wjw/gfxwj/201304/e33435ce0d894051b15490aa3219cdc4.shtml (accessed on 20 May 2018).
- Xiao-Ming, W.U.; Chun-Fu, W.U. Network pharmacology: A new approach to unveiling Traditional Chinese Medicine. Chin. J. Nat. Med. 2015, 13, 1–2. [Google Scholar] [CrossRef]
- Baptiste, B.; Karine, A.; Pierre, D.; Olivier, T. Network-based Approaches in Pharmacology. Mol. Inform. 2017. [Google Scholar] [CrossRef] [Green Version]
- Razmovski-Naumovski, V.; Huang, H.W.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Chemistry and Pharmacology of Gynostemma pentaphyllum. Phytochem. Rev. 2005, 4, 197–219. [Google Scholar] [CrossRef]
- Sá, M.d.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Sun, B.S. Extraction Yields and Anti-oxidant Activity of Proanthocyanidins from Different Parts of Grape Pomace: Effect of Mechanical Treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef]
- Upadhyay, P.; Tiwari, K.; Mishra, S.K. Pharmacognostic standardization of Asian folk medicinal plant Reinwardtia Indica Dumort. Int. J. Green Pharm. 2018, 12, 380–385. [Google Scholar]
- Wang, Y.; Wang, R.; Li, Y.; Sun, Y.; Song, C.; Zhan, Y.; Tan, L.; Liao, Y.; Meng, C.; Qiu, X.; et al. Newcastle disease virus induces G0/G1 cell cycle arrest in asynchronously growing cells. Virology 2018, 520, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Diptadip, D.; Kumar, S.R.; Sutapa, S.; Diana, K.; Muayad, A.; Varun, C.; Al, H.F.; Singh, U.P.; Kumar, S.R.; Erica, S. Sparstolonin B (SSnB) attenuates liver fibrosisvia a parallel conjugate pathway involving P53-P21 axis, TGF-beta signaling and focal adhesion that is TLR4 dependent. Eur. J. Pharmacol. 2018, 841, 33. [Google Scholar] [CrossRef]
- Peng, R.; Wang, S.; Wang, R.; Wang, Y.; Yuan, Y. Antifibrotic effects of tanshinol in experimental hepatic fibrosis by targeting PI3K/AKT/mTOR/p70S6K1 signaling pathways. Discov. Med. 2017, 23, 81–94. [Google Scholar] [PubMed]
- Wang, Q.C.; Wen, R.; Lin, Q.H.; Wang, N.; Lu, P.; Zhu, X.M. Wogonoside Shows Antifibrotic Effects in an Experimental Regression Model of Hepatic Fibrosis. Dig. Dis. Sci. 2015, 60, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Klaus, O.; Bart, V. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 2003, 4, 317–330. [Google Scholar] [CrossRef]
- Chu, W.; Rothfuss, J.; d’Avignon, A.; Zeng, C.; Zhou, D.; Hotchkiss, R.S.; Mach, R.H. Isatin sulfonamide analogs containing a Michael addition acceptor: A new class of caspase 3/7 inhibitors. J. Med. Chem. 2007, 50, 3751–3755. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Zhao, Y.; Fang, W.; Yang, W. Anticancer activity of Panax notoginseng extract 20(S)-25-OCH3-PPD: Targetting β-catenin signalling. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1074–1078. [Google Scholar] [CrossRef]
- Bi, X.; Xia, X.; Mou, T.; Jiang, B.; Fan, D.; Wang, P.; Liu, Y.; Hou, Y.; Zhao, Y. Anti-tumor activity of three ginsenoside derivatives in lung cancer is associated with Wnt/β-catenin signaling inhibition. Eur. J. Pharmacol. 2014, 742, 145–152. [Google Scholar] [CrossRef] [PubMed]











| No. | Retention Time | [M−H]+ | Error | Compounds |
|---|---|---|---|---|
| 1 | 8.37 | 1075.57105 | −1.49 | 10 |
| 2 | 9.19 | 1077.58650 | −1.30 | 14 |
| 3 | 9.90 | 1047.57184 | 2.57 | 15 |
| 4 | 10.09 | 1047.56959 | 4.72 | 1 |
| 5 | 10.19 | 1045.55885 | 0.03 | 18 |
| 6 | 15.21 | 1061.59252 | −2.20 | 12 |
| 7 | 15.30 | 1061.59273 | −2.39 | 13 |
| 8 | 23.39 | 1103.60267 | −1.74 | 3 |
| 9 | 26.67 | 883.50701 | −1.08 | 11 |
| 10 | 28.49 | 913.51883 | −2.42 | 8 |
| 11 | 30.38 | 751.46499 | −1.59 | 2 |
| 12 | 31.38 | 899.53926 | −2.11 | 7 |
| 13 | 31.76 | 911.50313 | −2.36 | 9 |
| 14 | 32.51 | 911.50094 | 0.04 | 6 |
| 15 | 33.13 | 955.52763 | −0.46 | 5 |
| 16 | 36.41 | 953.51526 | −3.87 | 4 |
| 17 | 36.92 | 953.51415 | −2.73 | 17 |
| 18 | 43.40 | 953.55206 | −4.33 | 16 |
| 19 | 47.01 | 969.58297 | −3.87 | 19 |
| Compounds | Linear Regression Data | Precision RSD (%) | Stability RSD (%) | |||
|---|---|---|---|---|---|---|
| Linearity Curve | R2 | Linear Range (mg/mL) | Intra-Day | Inter-Day | ||
| 1 | Y = 4 × 106x + 2.764 × 104 | 0.9992 | 0.01–1 | 1.86 | 1.38 | 2.47 |
| 2 | Y = 1 × 106x + 8.823 × 103 | 0.9994 | 0.01–1 | 2.08 | 2.57 | 2.20 |
| 3 | Y = 5 × 106x + 1.406 × 104 | 0.9993 | 0.01–1 | 1.57 | 2.02 | 1.93 |
| 4 | Y = 5 × 106x + 2.068 × 104 | 0.9991 | 0.01–1 | 1.62 | 1.54 | 1.52 |
| 5 | Y = 1 × 106x + 8.790 × 103 | 0.9994 | 0.01–1 | 1.05 | 1.99 | 1.76 |
| 6 | Y = 3 × 106x + 1.062 × 104 | 0.9991 | 0.01–1 | 1.13 | 1.07 | 1.35 |
| 7 | Y = 4 × 106x + 1.333 × 104 | 0.9991 | 0.01–1 | 1.25 | 0.98 | 1.88 |
| 8 | Y = 4 × 106x + 2.874 × 103 | 0.9991 | 0.01–1 | 1.37 | 2.88 | 1.06 |
| 9 | Y = 6 × 106x + 1.479 × 104 | 0.9989 | 0.01–1 | 1.67 | 1.49 | 2.50 |
| 10 | Y = 5 × 106x − 7.298 × 103 | 0.9992 | 0.01–1 | 1.27 | 2.38 | 2.04 |
| 11 | Y = 7 × 106x + 4.892 × 104 | 0.9992 | 0.01–1 | 1.94 | 1.94 | 1.69 |
| 12 | Y = 5 × 106x + 4.022 × 104 | 0.9992 | 0.01–1 | 2.11 | 1.74 | 2.51 |
| 13 | Y = 3 × 106x + 2.574 × 104 | 0.9991 | 0.01–1 | 2.04 | 2.03 | 2.15 |
| 14 | Y = 8 × 106x − 1.312 × 104 | 0.9991 | 0.01–1 | 2.68 | 2.22 | 2.81 |
| 15 | Y = 3 × 106x − 2.803 × 104 | 0.9991 | 0.01–1 | 2.53 | 1.25 | 1.44 |
| 16 | Y = 6 × 106x + 4.048 × 104 | 0.9992 | 0.01–1 | 1.68 | 2.79 | 2.85 |
| 17 | Y = 2 × 106x − 1.297 × 103 | 0.9992 | 0.01–1 | 1.79 | 1.30 | 2.66 |
| 18 | Y = 4 × 106x + 2.101 × 104 | 0.9990 | 0.01–1 | 2.05 | 2.74 | 1.57 |
| 19 | Y = 6 × 106x + 5.441 × 104 | 0.9992 | 0.01–1 | 1.98 | 1.83 | 1.09 |
| Compounds | t-HSC/Cl-6 | LO2 | Compounds | t-HSC/Cl-6 | LO2 |
|---|---|---|---|---|---|
| 1 | >200 | >200 | 11 | 43.6 ± 2.7 *** | >200 |
| 2 | 50.4 ± 1.4 *** | >200 | 12 | >200 | >200 |
| 3 | >200 | >200 | 13 | 105.6 ± 2.6 * | >200 |
| 4 | 126.4 ± 9.7 * | >200 | 14 | >200 | >200 |
| 5 | >200 | >200 | 15 | >200 | >200 |
| 6 | 99.9 ± 4.5 * | >200 | 16 | 44.7 ± 1.0 *** | >200 |
| 7 | 152.0 ± 7.4 | >200 | 17 | 66.1 ± 3.8 *** | >200 |
| 8 | 68.8 ± 3.2 *** | >200 | 18 | 58.0 ± 2.9 *** | >200 |
| 9 | 113.6 ± 7.8 * | >200 | 19 | 28.1 ± 2.0 *** | >200 |
| 10 | >200 | >200 | Silymarin b | 185.3 ± 10.1 | >200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shi, G.; Luo, Z.; Wang, J.; Wu, S.; Zhang, X.; Zhao, Y. Activity Components from Gynostemma pentaphyllum for Preventing Hepatic Fibrosis and of Its Molecular Targets by Network Pharmacology Approach. Molecules 2021, 26, 3006. https://doi.org/10.3390/molecules26103006
Zhang Y, Shi G, Luo Z, Wang J, Wu S, Zhang X, Zhao Y. Activity Components from Gynostemma pentaphyllum for Preventing Hepatic Fibrosis and of Its Molecular Targets by Network Pharmacology Approach. Molecules. 2021; 26(10):3006. https://doi.org/10.3390/molecules26103006
Chicago/Turabian StyleZhang, Yumeng, Guohui Shi, Zhonghua Luo, Jiewen Wang, Shao Wu, Xiaoshu Zhang, and Yuqing Zhao. 2021. "Activity Components from Gynostemma pentaphyllum for Preventing Hepatic Fibrosis and of Its Molecular Targets by Network Pharmacology Approach" Molecules 26, no. 10: 3006. https://doi.org/10.3390/molecules26103006