3.1. Characterization of D-Sorbitol
Polymorphism tendency is known to be an essential data for the development of an oral form. A modification of D-Sorbitol crystalline form can impact flowability of the entire formulation. [
46]. Therefore, all the D-Sorbitols selected for this study were analyzed by X-ray powder diffraction in order to verify their crystalline form. The five D-Sorbitols present an identical XRD pattern that proves their existence in the same crystalline form as showed by
Figure 2.
In order to observe the impact of the size and shape of the particles on the entire formulation extrusion process, five D-Sorbitol with different particle properties were selected.
PSD measurement of D-Sorbitol
Figure 3 highlight a monomodal particles distribution. CE and SI 150 have the lowest particles diameters with a D
v50 of 125 µm and 142 µm respectively. SI 200 have a D
v50 of 228 µm. GLT have the highest particles size with a D
v50 of 606 µm. CE D-Sorbitol presented a significant quantity of particles smaller than 50 µm in comparison to others D-Sorbitol even though the observation of the diameter in volume minimizes the presence of small particles. Sieved D-Sorbitol CE
T no longer has particles smaller than 45 µm which proves the effectiveness of the sieving process. This D-Sorbitol therefore presents higher D
v50 at 132 µm.
D-Sorbitol presented similar particle morphology as presented by SEM pictures in
Figure 4. Nevertheless, D-Sorbitol SI 150 and SI 200 had an identical particles morphology unlike other D-Sorbitol which were different in terms of particle morphology. The influence of D-Sorbitol CE sieving was highlighted by the SEM images. Indeed, CE presented particles smaller than 50 microns in contrary to CE
T.
Powder moisture can impact formulation flowability and product quality [
47,
48]. TGA was carried out to observe difference in mass loss between 20 °C and 115 °C corresponding to water loss. TGA graphs in
Figure 5 showed that below degradation temperature, the mass losses were similar, and no link were observed between the particle characteristics and the water losses between room temperature and 115 °C (
Table 4). D-Sorbitol showed a degradation starting at 200 °C. The HME and 3D printing process imposed a temperature of 80 °C on the components of the formulation. No degradation of D-Sorbitol was expected to occur during HME and 3D printing.
Particles shape and size are well known to be melting onset modifier [
49]. For organic compounds, it has been showed that the melting point lowers as the particle size decreases [
50]. Melting temperature is one of the characteristics which can influence HME settings. Therefore, it is important to assess the impact of the filling agent physical quality on the melting point.
D-Sorbitol melting onsets are observed in thermoanalytical curves presented in
Figure 6. Two groups in terms of melting onset were observed. The first group was formed by CE, CE
T and GLT. These powders presented a melting onset around 98 °C. The second group was formed by SI 150 and SI 200. These powders presented a melting onset around 96 °C (
Table 4). Each group seemed to present a similar particle’s shape indicating that it may played a role in the melting onset value contrary to the particles size which was not sufficiently far from a supplier to the other to have an influence on the melting onset.
Each of the D-Sorbitol used in the formulation had different particle sizes and shapes. These characteristics induce changes in flow properties [
51]. Flowability was an important bulk powder characteristic. Angle of repose, Carr’s index, Hausner ratio were used to express powders flowability [
51,
52].
It has been reported by Geldart et al., that angles of repose below 30° reflect a good flowability, for angles of 30–45° some cohesiveness, 45–55° true cohesiveness and >55° very high cohesiveness and very limited flowability [
52]. A Hausner ratio below 1.18 and a Carr’s index below 15 correspond to a good to excellent powder flow properties [
53].
It is well known that particle size and distribution are properties that influence powder flow [
54]. As observed
Table 5, CE D-Sorbitol present high cohesiveness compared to others D-Sorbitol which is highlighted by a high Carr’s index of 24.2 and an angle of repose of 30.2°. All powder flow indicators allow to conclude that CE is the most cohesive D-Sorbitol. Reducing particles size increases surface area and so surface cohesive forces which induce interactions and resulting in a more cohesive flow behavior [
55,
56,
57]. This phenomenon explained the difference of flowability between CE and CE
T as reflected by a Carr’s index of 24.2 for CE D-Sorbitol and 22.0 for CE
T D-Sorbitol. The particle sizes of D-Sorbitol SI 150, SI 200 and GLT were more important than those of D-Sorbitol CE and CE
T as showed previously which explained why their flow indicators reflected a better flowability.
3.2. Filaments Production and Characterization
In order to compare the thermal decomposition pattern of the formulations in state of powders, thermogravimetric analyses were carried out (
Figure 7). Samples’ weight loss revealed that all formulations do not contain significant amounts of water because of the 1% mass loss after reaching 115 °C. The degradation starts at 160 °C due to amiodarone hydrochloride degradation. Therefore, no degradation has been expected during HME and 3D printing because of a process temperature of 80 °C.
HME process gives white and opaque filaments due to the large quantity of crystalline products as shown in
Figure 8.
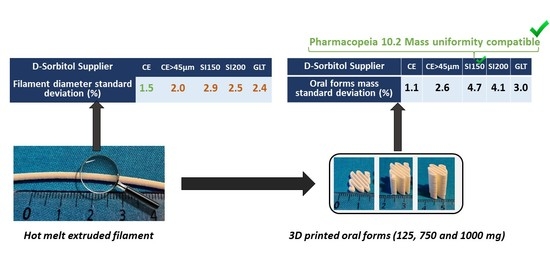
Five powder mixtures were tested in the extrusion process to produce pharmaceutical filaments. Depending on the formulation used, filament diameter measured by stericut vary. When a material comes out of the extrusion nozzle, the stericut measures its diameter and adjusts the belt speed to compensate any variations in flow. It has been identified that the delay between the diameter measurement and belt speed regulation induces a diameter variability. Moreover, a larger variability was observed when the flow presented significant oscillations. A filament diameter variability led to a mass variability which was of the same order of magnitude on the filament mass and 3D printed oral forms. Indeed, a filament with a larger diameter will contain, for a given length, more material.
All the D-Sorbitol powder flow indicators
Table 5 permitted us to conclude that D-Sorbitol CE present some cohesiveness. This product provided a formulation with a filament diameter standard deviation of 1.5% which was the best quality of filament obtained. Other D-Sorbitol gave a filament diameter variability of more than 2.0% but presented better powder flowability results. However, the study conducted on the formulated powders angle of repose did not make it possible to highlight significant differences in flow on the formulation (data not shown). Formulations’ flowabilities were equivalent, which was confirmed by an equivalent speed of regulation between 6.11 mm/s and 6.67 mm/s
Table 6. Stericut regulation speed were directly correlated to the flow rate of the powder entering the extruder and this flow rate was linked (at constant force feeder speed) to the powder flow properties. The absolute flow property of the formulation was not the parameter influencing standard deviation in all formulation. On the contrary, the flow variability induced variability in the HME flow rate and as a result in the filament diameter. It was therefore important to consider the overall flow of the formulation but also the flow variability in the system. As the different grades of D-Sorbitol have different melting temperature and the HME process only melted part of the D-Sorbitol during HME, the viscosity of the mixture containing D-Sorbitol could be different. However, sieved (CE
T) and unsieved (CE) D-Sorbitol have the same melting onset but do not exhibit the same variability in diameter (respectively 2% and 1.6%). D-Sorbitol GLT had the highest melting onset (98.4 °C) and SI 200 the lowest (95.7 °C), their coefficient of variation of diameter nevertheless remains close and respectively to 2.4% and 2.5%. This demonstrates that the thermal properties of D-Sorbitol are not the parameters modifying the RSD of filaments diameter.
D-Sorbitol particle size, morphology and filament diameter analysis allowed different observations. D-Sorbitol SI 150 and SI 200 had different particle sizes (Dv50 of 142 µm and 228 µm respectively) while possessing equivalent particle morphologies. However, the variation in filament diameter standard deviation remains equivalent to 2.9% for formulation B and 2.5% for formulation C. This means that the change in particle size does not significantly influence the standard deviation. The same observation can be made by comparing the GLT and SI 200 filament diameter standard deviation which are equivalent and respectively equal to 2.4% and 2.5%. GLT D-Sorbitol particles have a Dv50 of 606 µm in comparison to SI 200 that have a Dv50 of 228 µm. The filament diameter variability is not modified by those differences. Therefore, a particle size variation beyond 45 microns does not seem to significantly modify the standard deviation of the filament produced by HME. However, D-Sorbitol CE sieving increase filament diameter variability. From 1.6% of filament diameter variability when not sieved D-Sorbitol is used (formulation A) to 2% of filament diameter variability when sieved D-Sorbitol is used (formulation AT). Particles smaller than 45 microns in CE D-Sorbitol seem to have a stabilizing effect on the flow rate of the powder entering the extruder.
The PSD graph in
Figure 9 can explain impact of particles around 30 µm to obtain a constant powder flow. In fact, the PSD graph
Figure 9b represents the particle size distribution of the products contained in the formulation B respectively with respect to their quantity by mass in the formulation. Colloidal anhydrous silica is present in small quantities in the formulation, which is why it is not visible in the graph. Glycerol is a liquid plasticizer added into the formulation and no PSD measurement has been done on this product. All compounds’ particle distributions are added to obtain the green curve representing the particle size state of the theorical mixture. This mixture has particles of API centered around 12 microns as well as particles of D-Sorbitol and PEO centered around 200 microns. A small number of agglomerated particles is present around 1700 microns. The particle size distribution of the mixed and ground formulation is shown in black. The curve is equivalent to the theoretical mixture showing that the addition of glycerol and the grinding does not granulate the powder and does not significantly modify particles size distribution. The formulation particles size distribution highlight that the two particles population are separated by a hollow around 35 µm. Therefore, formulation B has a small number of particles around 35 microns. A similar PSD graph is presented in
Figure 9a with the formulation A, CE is the only D-Sorbitol to present a significant quantity of 35 µm particles. This could allow a particle size continuum which is supposed to induce a more constant flow by avoiding the formation of particle voids around 35 microns.
Formulated powder at the alimentation zone has been considered as the flow variability origin. However, it has been reported that formulation in state of melted product in HME can be the origin of some flow variability [
57]. Indeed, for linear polymers as polyethylene oxide, oscillation of flow volume and extrusion pressures are frequently observed and known as stick slip melt fracture [
58,
59]. This oscillation phenomenon occurs at high shear rate and can be diminish by the addition of filler into the polymer matrix [
60]. Filler size has been reported to influence the stick slip phenomena [
61]. In our case, the extruder was equipped with a conical corotative twin-screw system which allowed a gradual increase in pressure along the screws [
62]. This system allows a stable pressure during production. Moreover, flow rate and pressure are small in our hot melt extrusion system, so the shear rate is low. This oscillatory phenomenon that is sometimes present during polymer extrusion has therefore been ruled out.
Once the filament is produced, it can be used by 3D printer. During 3D printing process stream, the filament is subsequently fed into the FFF 3D printer. Inside the 3D printer, a feeding gear system drives the filament. Therefore, suitable mechanical properties of the filament are essential for the feeding process. The three-point bending test gives information about filament properties [
63,
64]. If it is brittle or too flexible, it cannot be used by 3D printing.
In a curve representing the force as a function of the displacement (
Figure 10), the first linear part of the curve represents an elastic behavior of the filament. The linear domain allows the obtention of filaments’ relative stiffness. When the curve is no longer linear, one passes from an elastic, and reversible strain to an irreversible strain. This transition is characterized by the elastic limit (pointed by the black arrow on
Figure 10). The maximum stress on the curve corresponds to the breaking point of the filament.
Different teams performed three-point bending analysis on pharmaceutical filaments [
26,
64,
65]. They used the three-point bending analysis without attaching the filament to the support. As the filament is a curved material that can be oriented differently depending on the sample, it was decided to attach it to two holding points. In addition, they used the point of maximum strength as a value characterizing filament property because of the difficulty to determine the elastic limit due to filament ductility. Given the large amount of nonmelted material contained in our formulation and the fact that the filament is attached to the support, it was decided to use the elastic domain limit as shown by the black arrow in
Figure 10 (obtained without offset). The latter is easily observable (on our formulations) and represents the point where the filament can present nonelastic comportment.
All of the extruded formulations were analyzed (
Figure 10) to obtain a force-distance curve. The samples all exhibit elastic then plastic behavior, ending with the breaking of the filaments. Extrudates exhibit ductile behavior represented by the appearance of plastic deformation in the force–distance curve.
Elastic distance is reported in
Table 7 to be greater than 1 mm for all formulations. D-Sorbitol grade and origin does not impact elastic strain of the filament produced. Elastic domain size is therefore not really influenced by size and particle shape.
However, filament stiffness is a parameter modified depending on the D-Sorbitol origin. The slope in elastic region is directly correlated to filament stiffness properties. A steep slope indicates a high filament stiffness. As shown in
Table 7, formulation B and C present an elastic domain slope of 2.0 N/mm and 2.1 N/mm which is equivalent. These formulations contain respectively SI 150 and SI 200 which are D-Sorbitol of identical morphology but different size. It shows that particle size in this case didn’t impact filament stiffness. The same conclusion can be done with A and A
T because they present different particles size and an equivalent stiffness of 1.2 N/mm and 1.0 N/mm. Filaments stiffness containing D-Sorbitol GLT (formulation D) is close to formulations B and C. However, D-Sorbitol GLT has a particle morphology closer to the D-Sorbitol used in formulations A and A
T.
Shao-Yun Fu and al, shows that composite modulus (linked to stiffness) is generally independent of particle size. However, when the particle size is decreased to a critical size of 30 nm, particle size will present an obvious effect on the stiffness [
66,
67]. This explains the small impact of D-Sorbitol size on filament stiffness. Moreover, two groups can be identified in terms of stiffness. Formulation B, C and D present a stiffness around 2.1 N/mm and formulation A and A
T a stiffness around 1.1 N/mm. It has been reported that a large aspect ratio is generally considered as mechanical properties modifier [
66]. It is supposed that particles morphology impact filament stiffness and should be considered when an unmelted filler is chosen.
Filaments were usable by 3D printing whatever the D-Sorbitol supplier. D-Sorbitol origin does not modify the elastic distance of the filaments produced as well as their macroscopic appearance. However, three-point bending analysis has showed through elastic domain slope that particle aspect seems to have an influence on filament stiffness.
3.3. Three-Dimensional Printing of Fast Disaggregation Oral Forms
Using filaments manufactured by HME, it is possible to produce oral forms with a dosage adapted to each patient by adjusting the size of the 3D printed object (
Figure 11).
Hot melt extrusion and 3D printing heats powder at 80 °C as shown in
Figure 12. It can induce change in thermal properties of components such as amorphization. Formulation B contains Merck D-Sorbitol, which is a European Pharmacopeia compliant excipient. This is why it has been analyzed by DSC in powder, filament and oral form as presented in
Figure 12. For all samples, three endotherms are observed corresponding respectively to PEO crystalline part melting (around 60–70 °C), D-Sorbitol melting (around 95 °C) and API melting (around 127 °C). Glycerol and silica are present in amounts too low to be identified with DSC analysis. A polymer melting point depression is observed for filament and oral form in comparison to PEO alone. Hot melt extrusion and 3D printing melts the polymer crystalline part which recrystallizes once the material has left the process. This implies a reorganization during recrystallisation inducing a lower intrinsic energy of the PEO matrices. D-Sorbitol and amiodarone hydrochloride present a melting point depression due to interaction with other formulation components [
68,
69,
70]. Considering the extrusion and 3D printing temperature, which is 80 °C, the drug is kept in a crystalline state in the filament and oral forms such as D-Sorbitol. However, a small proportion of D-Sorbitol is melted by the extrusion and 3D printing process. The low proportion of molten D-Sorbitol does not seem to have erased the impact of the particle’s morphology on the impact of the rigidity of the filaments presented previously. D-Sorbitol and API melting are not altered by the HME process as shown by identical endotherm on the thermoanalytical curves between oral form and filament in comparison to formulated powder in
Figure 12. Peak area is representative of the product quantity in the formulation. The area of PEO endotherm at 60 °C represents the crystalline quantity of PEO. The crystalline PEO quantity is equivalent between filament and powder with a respective area of 56 J/g and 59 J/g. The powder presents a higher quantity of crystalline PEO with a peak area of 73 J/g. This implies that the initial melting increases the proportion of amorphous PEO.
From the filaments manufactured by HME, oral forms were produced and weighed as presented in
Table 8. All filaments produced from different D-Sorbitol grade and suppliers allowed the manufacture of three different dosages demonstrating the capacity of dosage personalization by 3D printing from a filament. Three samples per assay were produced and mass RSD (%) was calculated. Results showed that formulation A allows the production of oral forms with the lowest mass variability identified by an RSD between 0.9% and 1.2%. All the other formulations allowed the manufacture of oral forms with a mass variability greater than 2%. The results showed that the variability in filament diameter impacted the mass variability of the printed oral forms. Indeed, the diameter of the filament conditioned the mass of the latter.
A supplier change can have a big influence on excipient physical properties. So, the use of D-Sorbitol in amounts of 37% by mass can have a big influence on processability or filaments and 3D-printed medicines’ quality [
22]. Results shows that sourcing is important in order to choose most suitable excipients to obtain better products quality.
In order to produce precise dose, a calibration on the oral forms masses was made according to the filament length used by the printer. Some teams used the volume of the modeled object as a calibration tool [
71]. However, the slicing operation can truncate parts of the volume according to the selected printing settings and therefore induce bias in the mass of the oral forms. That is why the length of the filament was used as a reference value by the printer to make the oral forms. To do so, three objects with different sizes were printed and the software provided a filament length needed. The manufactured objects were weighted, and a calibration curve drawn. The purpose being to link directly the dosage to the filament length.
European Pharmacopoeia 10.3 chapter 2.9.5 indicates that mass uniformity must be carried out on 20 randomly chosen samples. Tablet mass deviation from the average mass has to be smaller than 5 percent for tablets of 250 mg or smaller than 7.5 percent for tablets between 80 mg and 250 mg. A maximum of two tablets can be out of this range and none of them can deviate by more than twice that percentage [
24]. Regarding product quantity available in our lab, 10 samples with three different dosages were tested for this study using formulation B because of the pharmaceutical grade of D-Sorbitol SI 150. The mass uniformity study in
Table 9 highlights that the three dosages were within the specifications range of the European Pharmacopoeia. An oral form with a theoretical mass of 1000 mg gave 948.63 mg and one with a theorical mass of 750 mg gave 802.53 mg. These two samples were outside the deviation knowing that the European Pharmacopeia authorizes two for 20 samples. It was therefore possible to produce personalized oral forms with an acceptable standard deviation knowing that the variation in filament diameter was the origin of the variation in oral forms masses.
According to the hospital protocol, the oral form disintegration was performed in a syringe containing 5 mL of water under manual agitation. The objective is to disperse the amiodarone in water in order to integrate it into a compote or the content of a baby bottle. It has been observed by Patrojanasophon et al. that the size of the particles present in tablets can influence the disintegration time [
72]. This is why the disintegration time will be tested as a function of the D-Sorbitol incorporated in the formulations. As shown in
Table 10, all of the oral forms are disaggregated over three minutes in 5 mL of water. It has therefore been possible to produce oral forms of rapidly disintegrating amiodarone which can be dispersed in water. Whatever D-Sorbitol used, the disintegration time was unchanged. Particle size and filament diameter variability did not influence the release performance of the oral forms.