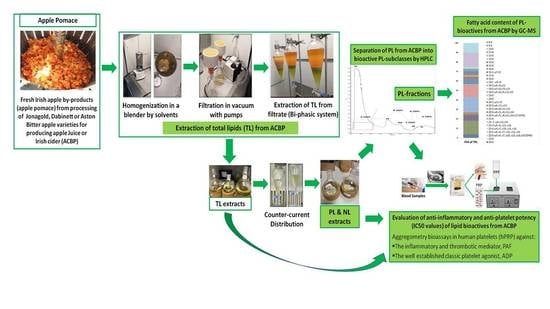
2.1. Yield Extraction of Lipids from ACBP and Fractionation by HPLC
The yields of extraction for the total lipids (TLs), neutral lipids (NLs), and PLs extracted from different ACBP samples are displayed in
Table 1 and are expressed as % percentage for each sample (g of lipids extracted per 100 g of sample). Similarly to previous studies in apples and other food by-products [
6,
7], the Bligh and Dyer extraction process [
18] in tandem with the Galanos and Kapoulas Counter Current Distribution technique [
19] were also chosen in the present study to extract and separate TL, NL, and PL extracts from the apple pomace for each ACBP. When these two methods are combined, they allow a simple and effective extraction and separation approach for obtaining dietary polar lipid bioactives as has been shown in several solid or liquid natural sources [
6,
7,
15,
16,
20,
21,
22,
23]. They have also shown excellent outcomes with respect to the yield of extraction of such bioactive PLs in each of these natural sources, with their fatty acid composition also being intact [
6,
7,
15,
16,
20,
21,
22,
23]. Such an increased efficacy for obtaining bioactive PLs from natural sources by combining these two methods further ensures very low to no loss of the bioactivities of bio-functional lipids during their extraction process, unlike other methods such as Soxhlet extraction, that uses high temperature and may thus detrimentally affect the fatty acid content of the extracted lipids [
24].
In addition, these methods were also chosen due to having been previously applied effectively for isolating and acquiring the bioactive PLs from the NLs from several similar plant-based sources and by-products for producing several beverages, such as apple cider, beer, wine, or even tea [
7,
15,
16,
21,
22,
23], in order to be able to observe their individual properties against inflammatory mediators in cell-models based bioassays.
According to the results shown in
Table 1, ACBP2 showed the highest yield for TL and PL which, however, was only statistically significant higher from that of ACBP3 that showed the lowest yield (
p < 0.05 for these comparisons). By contrast, the yield of extracted lipids from both ACBP2 (the highest) and ACBP3 (the lowest) did not statistically differ significantly when compared with the relevant intermediate yield of ACBP1 (
p > 0.05 for all these comparisons). Thus, even though the ACBPs assessed in this study originated from different apple sources varying in their tannin levels, this difference did not affect the yield of their TL, NL, and PL extracts.
In all ACBPs, the PLs made up approximately 55–85% of the TLs while the remaining 15–45% of the TLs seem to be its NL content, suggesting that the ACBPs of all these apple pomaces contain higher amounts of PL than NL. Nevertheless, it should also be stressed that according to the results shown in
Table 1, ACBP1 was the only apple pomace assessed that exhibited statistically significant higher yield of its PLs when compared to the relative yield of its NLs (
p < 0.05 for this comparison), suggesting that this variety contains, and thus can provide, more PLs than NLs, since approximately 75% of the TLs are PLs, while only the 25% of the TLs are NLs.
Furthermore, the yield of TL and PL extraction for all these ACBPs were found to be of significantly higher yield (2–10 times higher) than the relevant yields of extraction for TL and PL from the relevant apple-derived juices and cider products for each type of low, medium, and high in tannin apple varieties (Jonagold, Dabinett and Aston Bitter, respectively) [
7], but also when compared with the relevant yields for other beverages derived from plant/fruit sources, such as beer, wine, and tea [
7,
15,
21,
22,
23]. Such relative differences were also observed in analysis of PLs in apples, in which 10 times more PLs were found in the apple skin (that usually remains mostly in the apple pomace after squeezing processing) in comparison to the PLs found in the apple flesh (that is usually squeeze-processed for apple juice and cider production) [
25]. Thus, such results further suggest that the majority of the apple lipid content remains mainly in its apple pomace waste after processing for producing apple juice and cider products.
Taking into account also that the yields of extraction for both TLs and PLs for all these ACBPs were similar to the yields of highly bioactive PLs found in other foods, such as several marine sources and their relevant by-products [
6,
20], further suggests that all these ACBPs and especially ACBP1 and ACBP2 are also good sources for acquiring bio-functional PLs. However more studies are needed, and especially in extraction procedures using environmentally friendly solvents and techniques, for fully evaluating the potential for high yield of food grade extracted bioactive PLs from each type of ACBP.
Since the PL extracts of all ACBPs showed potent anti-inflammatory and antithrombotic properties against PAF and anti-platelet effects against ADP, the polar compounds of these PL extracts from all ACBPs were further separated into six main fractions/PL subclasses by HPLC analysis, as previously described [
7]. For all these PL extracts, HPLC analysis was performed using a wavelength of 208 nm, where double bonds in lipids and, thus, the lipids themselves can be detected, but also at 280 nm, where phenolic groups usually are detected. The individual fractions represented the respective subclasses of polar compounds within the PL extracts by utilizing specific standards (
Table 2).
More specifically, the separation of the six PL fractions shown in
Table 2 of the PL extracts for each one of the ACBP was performed according to their retention times in comparison with the retention times obtained for several standards of phenolic compounds (detected at 280 nm) and PL molecules (specific glycerol/sphingosine-based phospholipids, glycolipids, and sulfatides/sulfolipids), detected at 208 nm, by applying a specific gradient mobile phase in a normal phase column for this HPLC analysis, which has been previously described in detail during a similar HPLC analysis of the PL extracts obtained from the apple juices and cider products from the same apple varieties (Jonagold, Dabinett and Aston Bitter, respectively) [
7]. A representative chromatogram of such analysis is shown in
Figure 1.
According to this HPLC analysis and based on the retention times of the relative standards analyzed, as shown in
Table 2, fraction 1 compounds comprising of molecules with phenolic groups were eluted at 0–15 min, polar sphingo-based glycolipid compounds were eluted in fraction 2 at 15–30 min, PC molecules of PLs were eluted in fraction 3 at 30–45 min, sphingomyelin (SM) and some sulfo- and glycerol-based glycolipid compounds such as monogalactosyldiglyceride (MGDG) and digalactosyldiglyceride (DGDG) were eluted in fraction 4 at 45–60 min, while fraction 5 contained PE molecules of PLs that where eluted at 60–75 min, and the remaining fraction 6 was comprised of phosphatidylinositol (PI), phosphatidylserine (PS), and of remnants of other more polar lipid compounds such as phosphatidylglycerol (PG), eluted at 75–90 min.
The presence of such PL subclasses in apple pomace observed in the present study is also in accordance with previously reported outcomes in apple skin and apple flesh [
25], but also in apple juice and cider [
7]. From weighing the PL fractions obtained from such HPLC analysis of 1 mg of the PL extracts from all ACBP, very similar percentage values for each fraction were observed between the different ACBP apple pomaces, with the exception of fraction 1 that contains phenolics, the percentage of which was increased from ACBP1 (low in tannins) to ACBP3 (high in tannins). More specifically, approximately 35–45% of the PLs in all apple pomaces (ACBP1, ACBP2, and ACBP3) was found to be PC, 20–30% was PE, 15–25% was MGDG, DGDG, and all the other lipids eluted in fraction 4 such as SM and sulfatides, while only 10–25% were phenolics eluted in fraction 1 (approximately 10% in ACBP1, 10–20% in ACBP2, and 20–25% in ACBP3) and 5–15% PI, PS, and other polar lipid compounds like PG that were eluted in fraction 6 as well as 5–10% of lipids eluted in fraction 2. Such relative % composition of the PL fractions within the PL extracts observed in all apple pomaces of the present study are similar to the ones previously observed in both apple skin and flesh [
25]. However, more sophisticated lipidomic approaches, based on modern MALDI-TOF LC–MS, are needed for quantifying and structurally elucidating all PL bioactive molecules present in each PL subclass from the PLs of all ACBPs to support the above findings, as was previously described for the grape pomace and Irish ale beer cases [
16,
21].
Furthermore, all TL, NL, and PL extracts for each ACBP apple pomace and each of the six HPLC-derived PL fractions were further assessed in the platelet aggregometry bioassay for their ability to inhibit platelet aggregation induced either by the PAF-related inflammatory and thrombotic pathways or by the well-established platelet activation agonist, ADP (for PL), while the fatty acid composition of all PL extracts and of the most bioactive HPLC-derived PL subclasses were further elucidated by GC–MS analysis.
2.2. Anti-Inflammatory and Anti-Platelet Properties of the ACBP Lipid Extracts (TL, NL, and PL) and HPLC-Derived Bioactive PL Subclasses
The biological activities of the TL, NL, and PL extracts from the three types of apple pomaces, ACBP1, ACBP2, and ACBP3, were evaluated by acquiring their putative anti-inflammatory and anti-platelet potency against human platelet activation and aggregation induced by the inflammatory and thrombotic mediator PAF as previously described [
6,
7,
20,
23]. The results obtained from these bioassays in human platelets are shown in
Figure 2, in which the anti-inflammatory and antithrombotic potency of the bioactive ACBP lipid extracts (TL, NL, and PL) are expressed as means of their IC
50 (half-maximal inhibitory concentrations) values in µg of ACBP TLs, NLs, and PLs in the aggregometer cuvette that causes 50% inhibition of PAF-induced platelet aggregation. It should also be stressed that the lower the IC
50 value for a lipid bioactive against the PAF-induced platelet aggregation, the more superior its inhibitory effect against the PAF pathways of inflammation and thrombosis.
All lipid extracts exhibited potent anti-inflammatory and antithrombotic activities against the PAF pathway of aggregation of human platelets, with the PLs in each ACBP sample showing the most potent inhibitory effects against the PAF pathway of platelet aggregation, with IC
50 values of their anti-PAF activities being approximately within the range of 70–180 μg (
Figure 2B), which were found to be statistically significant lower (and thus more bioactive) than the relative anti-PAF IC
50 values of both TLs and NLs for each ACBP shown in
Figure 2A (
p < 0.05 for all these comparisons). On the other hand, the NL extracts of all ACBP samples assessed showed the lowest inhibition against the PAF pathway of platelet aggregation, with IC
50 values of these anti-PAF effects being approximately within the range of 300–700 μg (
Figure 2A), which were found to be statistically significant higher (and thus less bioactive) than the relative IC
50 values of both PLs and TLs for each ACBP (
p < 0.05 for all these comparisons). Thus, the TL extracts in all ACBP samples showed intermediate but considerable potency against PAF-induced human platelet aggregation, with the IC
50 values of these inhibitory effects against the PAF pathway being approximately within the range of 180–270 μg (
Figure 2A). The intermediate inhibitory action observed for the TL extracts of each one of the three ACBPs against PAF-induced platelet aggregation seem to be derived by the combination of their more-active PL content and less-active NL content.
These results are in accordance with previously reported ones for similar differences observed in the anti-inflammatory properties against the PAF pathway of human platelet aggregation between the TL, NL, and PL extracts of apple products (apple juice and cider) [
7] which were produced from the low (Jonagold), medium (Dabinett), and high (Aston Bitter) in tannin apple varieties that are relevant to the ACBP apple varieties assessed, and were extracted and separated with the same methodology applied in the present study. More specifically, in both the apple juice and cider products of these three apple varieties, the PL extracts were found again to be more bioactive against PAF [
7], as in the case of their relative ACBP apple pomaces observed in the present study. In addition, the differences observed in these anti-PAF effects between the TL, NL, and PL extracts in each ACBP sample are also in accordance with previously reported similar differences for the anti-PAF bioactivities of the TL, NL, and PL extracts of other beverages and plant/fruit-derived sources and by-products for beverage production, such as wine, beer, and tea [
15,
16,
21,
22,
23], which were also extracted and separated with the same methodology applied in the present study.
It should also be stressed that no statistically significant difference was observed when the anti-PAF potency (IC50 values) of the PL extracts from ACBP1 were compared to the relevant ones for the PL extracts of the medium in tannin ACBP2 and the high in tannin ACBP3 (p > 0.05 in all these comparisons of the different PL bioactivities). This result further suggests that the potent anti-inflammatory and anti-thrombotic potency of the PL extracts in all ACBP apple pomaces seem to not be associated to their tannin content. Similarly, no differences were observed when comparing the anti-PAF bioactivities of all TL extracts from these three ACBPs within each other (p > 0.05 in all these comparisons of the different TL bioactivities), or even when comparing the NL anti-PAF effects from these three ACBPs (again p > 0.05 in all these comparisons of the different NL bioactivities), which further support the notion that the observed anti-PAF bioactivities of the ACBP lipid extracts is irrelevant to their tannin content. This finding may also be associated to a possible migration and loss of tannins (as more hydrophilic compounds) at the hydroalcoholic phase during the Bligh and Dyer extraction process. However, this assumption requires further research in order to be confirmed, and especially such comparisons in food grade lipid extracts of ACBP with different tannin contents using environmentally friendly solvents and food grade approaches.
Nevertheless, the PL from all ACBP were found to be similar to less active and within the same order of magnitude as the PAF-associated inflammatory and thrombotic pathways when compared to the anti-PAF bioactivities of the PL from their relative apple products (apple juice and cider) [
7], which were also produced from the same apple varieties used for the ACBP and were also extracted and separated with the same methodology applied in the present study and also from other beverages and plant/fruit-derived sources and by-products for beverage production, such as grapes, wine, yeasts, and winery by-products (grape pomace) [
15,
16], beer and brewery by-products [
21,
22], tea [
23], and olive pomace [
26].
Since the PLs were the lipid compounds in all three ACBP apple pomaces with the most potent anti-PAF effects in human platelets, and in order to evaluate their overall putative anti-platelet beneficial properties, the ACBP-derived PL extracts were also further assessed against the platelet aggregation induced by a classic and well-established platelet agonist, ADP, which activates platelets through pathways other than those of platelet aggregation induced by PAF [
1,
2,
3,
4], as previously described [
6,
7,
23]. The results obtained from these bioassays in human platelets are also shown in
Figure 2B, while the anti-platelet potency of the ACBP PL bioactive extracts were again expressed as means of their IC
50 values (µg of ACBP PL in the aggregometer cuvette that causes 50% inhibition of ADP-induced platelet aggregation). Once more, the lower the IC
50 value for a lipid bioactive against ADP-induced platelet aggregation, the more superior its inhibitory effect against the ADP-associated thrombotic pathways.
The PL extracts from each ACBP showed considerable but significantly lower anti-platelet properties against the ADP pathway of platelet aggregation, with higher IC
50 values for their anti-ADP activities that were within the range of 300–700 μg (
Figure 2B), which were also found to be statistically significant higher (and thus less bioactive) than the relative anti-PAF potency (IC
50 values) of these PL extracts in each ACBP (
p < 0.05 for all these comparisons). These results are in accordance with previously reported ones observed in dietary PLs and further suggest that the bioactive PL extracts of ACBP apple pomaces also have higher specificity against the PAF-associated inflammatory and thrombotic pathways rather than against other platelet-activation pathways induced by classic platelet agonists, such as ADP. Similar superior anti-PAF properties of several dietary PLs, such as the aforementioned ones observed for the PL extracts of all ACBP apple pomaces assessed in the present study, seem to be associated with their structural resemblance to PAF and the subsequent structure–activity relationships of their antagonistic effects against the binding of PAF to the unique for PAF G-coupled protein cell membrane receptor (PAF-R) [
1,
2,
3,
4,
5,
6,
7,
15,
16,
20,
21,
22,
23,
26].
Such an observed higher efficacy of the PL extract from the ACBP1 apple pomace, low in tannin, against the inflammatory PAF pathway in comparison to its lower anti-ADP effects has not been previously observed in bioactive PLs from apple products (apple juices and cider products) from the same apple variety (Jonagold) [
7]. In contrast, in both apple juice and cider from apples low in tannin, the anti-PAF efficacy of their PL was similar to their efficacy against the ADP pathway [
7]. This outcome further suggests that some PL bioactives of the low in tannin apple variety (Jonagold), that possess strong anti-ADP efficacy, seem to migrate to the apple products (apple juice and cider) during processing and, to a lesser extent, within the relevant ACBP remnants/wastes of such processing. Nevertheless, the anti-PAF activities of the PL extracts from both the apple products (apple juice and cider) and their ACBP of the low in tannin apple variety (Jonagold), were of similar potency (within the same order of magnitude), suggesting that the PL bioactives of this apple variety with strong anti-PAF efficacy seem to migrate equally to its apple products (apple juice and cider) and to the relevant ACBP remnants/wastes during processing.
Moreover, no statistically significant difference was observed between these low anti-ADP effects of the PL from these ACBP apple pomaces, which further support the notion that apart from the anti-inflammatory potency against PAF, the anti-platelet potency of the bioactive ACBP lipids against the ADP-pathway is also not associated with their tannin content.
Since the PL extracts of all assessed ACBPs were the most bioactive against the inflammatory PAF pathway, as mentioned earlier, they were further separated into molecular subclass fractions using HPLC analysis, performed using a wavelength of 208 nm, where double bonds in lipids and, thus, the lipids themselves can be detected, but also at 280 nm, where phenolic groups are usually detected. All the PL subclasses/fractions obtained from the HPLC analysis of each ACBP PL extract were also assessed for their putative bioactivities to inhibit the PAF-induced aggregation of human platelets. The results obtained from these bioassays in human platelets are shown in
Figure 3, in which the anti-inflammatory potency of the ACBP-derived bioactive PL subclasses are again expressed as the means of their IC
50 values (µg of ACBP-derived bioactive PL compound in the aggregometer cuvette that causes 50% inhibition of PAF-induced platelet aggregation). Similarly, the lower the IC
50 value for a lipid bioactive compound (PL subclass) against the PAF-induced platelet aggregation, the more superior its inhibitory effect against the PAF-associated inflammatory and thrombotic pathways.
Differently than the overall PL extracts that showed potent anti-inflammatory properties against the PAF pathway for all ACBPs (
Figure 2), the anti-inflammatory potency for all the HPLC-derived ACBP PL fractions (IC
50 values shown in
Figure 3) varied depending on the different PL subclasses present in each PL fraction. Within all these assessed PL fractions, lipid fraction 3 (F3), in which the bioactive PC molecules were eluted, exhibited the most potent anti-PAF effects when compared to all the other PL fractions in all ACBP apple pomaces (
p < 0.05 for all these comparisons). These results are in accordance with those previously reported for the relative lipid fraction 3 containing PC molecules of the PLs from apple juice, which were derived from the same apple varieties (Jonagold, Dabinett and Aston Bitter) by following similar experimental methodology [
7], further suggesting that the PC bioactives of these apple varieties with strong anti-PAF efficacy seem to migrate equally to its apple juice product and to the relevant ACBP apple pomace remnants/wastes during processing.
In addition, these results are also in accordance with those observed in relevant studies in other healthy foods such as oily fish (salmon) [
20], beverages such as beer [
21], and other dietary sources such as microorganisms of biotechnological interest in the food industry, such as microalgae (
Spirulina) [
27] and bioethanol-producing bacteria (
Zymomonas mobilis) [
28], in which, again, the PL fractions containing bioactive lipid molecules belonging to the PC family were the most potent anti-inflammatory lipid subclass against the PAF pathway in several models of inflammation and platelet aggregation [
1,
20,
21,
27,
28]. It should also be stressed that such dietary bioactive PC molecules are constitutionally more abundant in small, dense HDL cholesterol, where they preferably bind, while these small, dense HDL lipids enhance HDL functionality and increase HDL levels, embodying cardio-protective properties, antithrombotic potency, and antioxidant protection in cells and against LDL oxidation [
1,
29].
Moreover, the PL subclass corresponding to the bioactive PE molecules that were eluted in lipid fraction 5 (F5) also showed an intermediate and considerably potent anti-inflammatory bioactivity against the PAF pathway, which was statistically less potent than the anti-PAF effects of the relative PC molecules, but more potent than all the other lipid fractions in all ACBPs, with the exception of the lipid fraction 4 (F4) for the ACBP3 that also showed a considerable strong anti-PAF bioactivity. Similar dietary PE bioactives from other food sources, such as those eluted in lipid fraction F5 of the ACBPs, were also reported to possess strong anti-PAF effects in several cells, including platelets [
1,
20,
21]. It should be mentioned that within the PL fraction F4, bioactive MGDG, DGDG, SM and some sulfatides are usually eluted, which were also previously observed in Irish ale beer with strong anti-platelet effects, while they have also shown strong anti-inflammatory and antitumor properties [
21].
On the contrary, very low anti-PAF potency was found in the lipid fraction 2 (F2) in which sphingo-based glycolipids were eluted in all ACBP, and especially in ACBP2, that exhibited the lowest anti-PAF effects. These results suggest that such sphingoglycolipid bioactives seem to be less bioactive or in lower amounts in all ACBPs in comparison to all the other lipid bioactives eluted in the other lipid fractions of ACBP PLs.
Furthermore, in all ACBPs, the lipid fraction 6 (F6), which contains the more polar PLs (i.e., PIs, PSs, etc.), was found to have stronger anti-PAF bioactivities than the lipid molecules of F2. In contrast, PLs in F6 were less bioactive against the PAF pathway when compared to either the PC fraction F3 or the PE fraction F5. Nevertheless, the anti-PAF effects of the PLs in F6 were comparable to those found in F4 which, as aforementioned, contains SM, glycerol-based glycolipids such as MGDG and DGDG, and sulfatides only in ACBP1 and ACBP2. These bioactivities in both F6 and F4 were also similar to the anti-PAF effects found in fraction 1 (F1), which contains phenolic compounds/functional groups only in the case of ACBP2 and ACBP3. Exceptions to the above are F4 from the PLs of ACBP3 which, as aforementioned, exhibited stronger anti-PAF effects comparable to those of the PE molecules of F5, but also the phenolics of F1 in the low in tannin ACBP1 that showed very low anti-PAF potency.
Interestingly, apart from F1 of ACBP1, the stronger anti-PAF bioactivities observed in the phenolics eluted in fraction 1 of both ACBP2 and ACBP3 were found to be approximately 3 times more potent than those previously reported for the relative apple juices from intermediate and high in tannin Dabinett and Aston Bitter apple varieties, respectively. This result seems to be associated with the higher content of tannins in these apple varieties as mirrored by the relatively higher % composition of F1 in ACBP2 and ACBP3 in comparison to that of the low in phenolic content of F1 from the low in tannin ACBP1, indicating that some more bioactive phenolics remain mainly in the apple pomace after processing for apple juice and cider production, especially in the cases of ACBP2 and ACBP3. However, more studies are needed to fully elucidate the relative anti-inflammatory and anti-platelet contribution of the phenolics eluted in F1 of the PL from apple pomace.
Overall, the strong anti-inflammatory potency observed in the PL extracts of all ACBP apple pomaces against the PAF pathway seem to be derived by the interplay of all these lipid bioactives eluted in their HPLC-derived lipid fractions which, on some occasions, have also been found to act synergistically against PAF-induced inflammatory platelet aggregation, leukocyte activation, endothelial dysfunction, and LDL oxidation, thus reducing the risk for CVD and other inflammation related chronic disorders [
1]. Nevertheless, more studies are needed in order to elucidate such potential for the ACBP lipid bioactives that will further enhance the use of apple pomaces as a sustainable source for such PL bioactives.
2.3. Fatty Acid Composition of Bioactive PL from ACBP by GC–MS
The fatty acid composition of the PL extracts and of their most bioactive PC subclass from all ACBP apple pomaces was elucidated by GC–MS analysis, and the results are displayed in
Table 3 and
Table 4, respectively. All PL extracts from these three ACBPs were found to be rich in polyunsaturated fatty acids (PUFAs), followed by lower amounts of saturated fatty acids (SFA) and the less abundant monounsaturated fatty acids (MUFA) (
Table 3). More specifically, all bioactive ACBP-derived PL extracts contained high amounts of the most abundant essential omega-6 (n-6) PUFA, linoleic acid (LA) (C18:2 c9, 12 n-6), followed by the essential omega-3 (n-3) PUFA, alpha linolenic acid (ALA) (C18:3 c9,12,15 n-3), and by much less but considerable amounts of other bioactive n-3 PUFAs such as eicosapentaenoic acid (EPA) (C20:5 c5,8,11,14,17 n-3), docosapentaenoic acid (DPA) (C22:5 c7,10,13,16,19 n-3), and docosahexaenoic acid (DHA) (C22:6 c4,7,10,13,16,19 n-3). In all PL extracts of the three ACBPs, hexadecanoic acid (C16:0) was the most abundant SFA, while the most abundant MUFA was oleic acid (C18:1 c9), but in considerably lower amounts than the aforementioned SFA and PUFA content of PL from ACBP. Similar fatty acid compositions were also obtained from the GC–MS analysis of the most bioactive PC lipid fractions of these PL extracts in all ACBPs (
Table 4).
These results are in accordance with the previously reported fatty acid content of the bioactive PL extracts of apple products (apple juice and cider) from the same apple varieties (Jonagold, Dabinett, Aston Bitter) and of the PC bioactives of the PL extract of apple juices from the low in tannin Jonagold apple variety [
7], and in apples in general [
25], while they again further suggest that such PL bioactives rich in n-3 PUFA seem to migrate equally to its apple juice/cider products and to the relevant ACBP apple pomace remnants/wastes during processing.
Interestingly, it has been previously proposed that plant sources do not contain the long chain n-3 PUFA EPA, DPA, and DHA due to lack of appropriate enzyme machinery for producing them from ALA and LA, yet Guil et al. have reported the presence of low amounts of both EPA and DHA in several natural plants [
30], which was also recently observed in tea PL bioactives [
23] but also in the ACBP-derived PL bioactives assessed in the present study and in the PL bioactives of apple products (apple juice and cider) from the same apple varieties (Jonagold, Dabinett, and Aston Bitter) [
7], while apple-derived rhamnogalacturonan II, the most structurally complex segment of the 10% of apple pectin, and its unusual sugar-based compounds have also been reported to contain DHA as determined by GC–MS of their trimethylsilyl-esters O-methyl glycosides [
31].
In addition, the presence of such essential n-3 PUFA (mostly ALA, but also much lower but considerable amounts of EPA, DPA, and DHA) bound in the bioactive PL extracts of the ACBP, and especially in their most potent PC bioactives, further support their anti-inflammatory potency and provide a rationale for their strong anti-PAF effects as previously described in several other bioactive dietary PLs and PCs of natural origin, and especially in PL bioactives from healthy food sources [
1,
6,
7,
20,
21,
22,
23]. Such dietary PLs rich in n-3 PUFA have been found to inhibit platelet aggregation induced by the inflammatory and thrombotic mediators, PAF and thrombin, but also by classic well-established platelet agonists such as collagen and ADP [
1,
6,
7,
20,
21,
22,
23] as was also observed in the present study for the rich in n-3 PUFA bioactive PL extracts of ACBPs and their potent PC bioactives. Nevertheless, apart from the bioactivities observed on the PL bioactives, the n-3 PUFA content of these PLs and especially of the PC bioactives has, on its own, several beneficial bio-functionalities, especially when released from these PL in cells by specific cytoplasmic phospholipases A2 (PLA2) enzymatic activities. For example, the PLA2-based release of n-3 PUFA from such bioactive PLs in cell membranes and/or lipoproteins facilitate the production of anti-inflammatory eicosanoids that act antagonistically to other inflammatory and thrombotic eicosanoids (prostaglandins, leukotrienes, and thromboxanes) usually produced by n-6 PUFA such as arachidonic acid and LA [
32]. The latter further supports the health benefits derived from the aforementioned n-3 PUFA (ALA, EPA, DPA, and DHA), while healthy dietary patterns based on these n-3 PUFA have shown strong preventative benefits against several chronic disorders, such as in a Mediterranean diet enriched in ALA for the secondary prevention of coronary heart disease [
33].
Subsequently, a relative index of the anti-inflammatory potency of dietary lipid bioactives, such as the bioactive PLs rich in n-3 PUFA that are present in healthy foods and diets, is the n-6/n-3 PUFA ratio, for which it has been proposed that the lower the value for this ratio, the better the preventative anti-inflammatory benefits against several inflammation- and platelet aggregation-related chronic disorders, and vice versa [
32]. In the PL extracts of all ACBPs, the n-6 PUFA content was not so much higher than their n-3 PUFA content, which resulted in favorable low values of the n-6/n-3 PUFA ratio of these bioactive ACBP PLs within the range of 1.4–2.9 (
Table 3), while for the PC bioactives, this ratio ranged at much lower values of approximately 1.1–2.0 (
Table 4) that were lower than previously reported values for this ratio in apples [
34] and apple juices [
7]. The low values of the n-6/n-3 PUFA ratio observed in ACBP-derived PL bioactives and especially in their PC bioactives are usually observed in healthy foods and diets, while they are also much lower than the values above 15/1 for this ratio that are usually observed in unfavorable Western-style/Westernized foods and diets [
32]. The above findings of the favorable low values for the n-6/n-3 PUFA ratios in PL bioactives of all the tested ACBPs, and especially in their bioactive PC subclasses, further support the potential anti-inflammatory and cardio-protective properties of the ACBP-derived PL bioactives.