Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells
Abstract
:1. Introduction
2. Results and Discussion
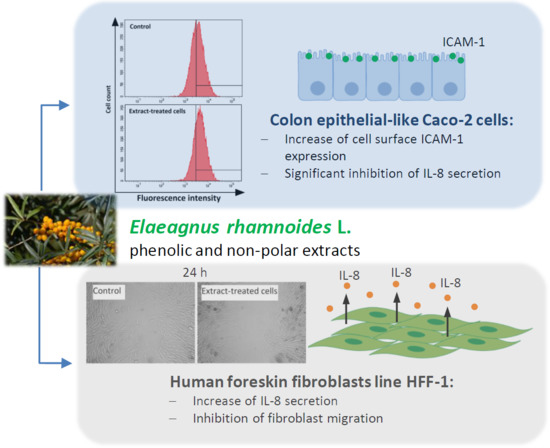
2.1. The Effect of Fractionated E. rhamnoides Extracts and Reference Compounds on Caco-2 Cells
2.2. The Impact of Fractionated E. rhamnoides Extracts and Reference Compounds on HFF-1 Cells
3. Materials and Methods
3.1. Chemicals and Media
3.2. Plant Material
3.3. Preparation of Fractionated Sea Buckthorn Extracts and Phytochemical LC-MS Analyses
3.4. Preparation of the Solutions of Fractionated Sea Buckthorn Extracts and Reference Compounds
3.5. Cell Cultures
3.6. ELISA Assay for Assessment of IL-8, MIP-1α, and TNF-α Production
3.7. Scratch Assay to Determine of the Fibroblasts Migration
3.8. Assessment of ICAM-1 Expression on Caco-2 Cells Using Flow Cytometry
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brglez, M.E.; Knez, H.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food. Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food. Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Olas, B. Berry phenolic antioxidants—Implications for human health? Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef]
- Mendes, L.F.; Gaspar, V.M.; Conde, T.A.; Mano, J.F.; Duarte, I. Flavonoid-mediated immunomodulation of human macrophages involves key metabolites and metabolic pathways. Sci Rep. 2019, 9, 14906. [Google Scholar] [CrossRef] [Green Version]
- Che, D.N.; Cho, B.O.; Kim, J.S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Effect of luteolin and apigenin on the production of IL-31 and IL-33 in lipopolysaccharides-activated microglia cells and their mechanism of action. Nutrients 2020, 12, 811. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.Y. Metabolism of green tea catechins: An overview. Curr. Drug. Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef] [PubMed]
- Botting, R.A.; Haniffa, M. The immunological network in the developing human skin. Immunology 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Radloff, J.; Cornelius, V.; Markov, A.G.; Amasheh, S. Caprate modulates intestinal barrier function in porcine peyer’s patch follicle-associated epithelium. Int. J. Mol. Sci. 2019, 20, 1418. [Google Scholar] [CrossRef] [Green Version]
- Schwerdtfeger, L.A.; Tobet, S.A. Vasoactive intestinal peptide regulates ileal goblet cell production in mice. Physiol. Rep. 2020, 8, e14363. [Google Scholar] [CrossRef] [Green Version]
- Toncic, R.J.; Jakasa, I.; Hadzavdic, S.L.; Goorden, S.M.; Vlugt, K.J.; Stet, F.S.; Balic, A.; Petkovic, M.; Pavicic, B.; Zuzul, K.; et al. Altered levels of sphingosine, sphinganine and their ceramides in atopic dermatitis are related to skin barrier function, disease severity and local cytokine milieu. Int. J. Mol. Sci. 2020, 21, 1958. [Google Scholar] [CrossRef] [Green Version]
- Larmo, P.; Alin, J.; Salminen, E.; Kallio, H.; Tahvonen, R. Effects of sea buckthorn berries on infections and inflammation: A double-blind, randomized, placebo-controlled trial. Eur. J. Clin. Nutr. 2008, 62, 1123–1130. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and nonpolar fractions of Elaeagnus rhamnoides (L.) A. Nelson extracts as virulence modulators —in vitro study on bacteria, fungi, and epithelial cells. Molecules 2018, 23, 1498. [Google Scholar] [CrossRef] [Green Version]
- Olas, B.; Żuchowski, J.; Lis, B.; Skalski, B.; Kontek, B.; Grabarczyk, Ł.; Stochmal, A. Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A. Nelson fruits. Food. Chem. 2018, 247, 39–45. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S.; et al. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: Evidence from studies with heat-stressed Caco-2 cells, C. elegans and growing broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of interleukin-8 and neutrophil extracellular trap (net) formation in the tumor microenvironment: Is there a pathogenic role? J. Immunol. Res. 2019, 2019, 6252138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, D.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Updat. 2007, 2, 20–35. [Google Scholar] [CrossRef] [Green Version]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of quercetin, a polyphenol, on blood pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef] [Green Version]
- Michalski, J.; Deinzer, A.; Stich, L.; Zinser, E.; Steinkasserer, A.; Knippertz, I. Quercetin induces an immunoregulatory phenotype in maturing human dendritic cells. Immunobiology 2020, 151929, in press. [Google Scholar] [CrossRef]
- Kim, I.B.; Kim, D.Y.; Lee, S.J.; Sun, M.J.; Lee, M.S.; Li, H.; Cho, J.J.; Park, C.S. Inhibition of IL-8 production by green tea polyphenols in human nasal fibroblasts and A549 epithelial cells. Biol. Pharm. Bull. 2006, 29, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic. Dis. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chow, M.P.; Huang, W.C.; Lin, Y.C.; Chang, Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: Structure-activity relationships. Mol. Pharmacol. 2004, 66, 683–693. [Google Scholar] [PubMed]
- Freese, R.; Vaarala, O.; Turpeinen, A.M.; Mutanen, M. No difference in platelet activation and inflammation markers after diets rich or poor in vegetables, berries and apple in healthy subjects. Eur. J. Nutr. 2004, 43, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkita, M.; Hayashi, H.; Ito, K.; Shigematsu, N.; Tanaka, R.; Tsutsui, H.; Matsumura, Y. Preventive effects of grape extract on ischemia/reperfusion-induced acute kidney injury in mice. Biol. Pharm. Bull. 2019, 42, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Skalski, B.; Stochmal, A.; Żuchowski, J.; Grabarczyk, Ł.; Olas, B. Response of blood platelets to phenolic fraction and non-polar fraction from the leaves and twigs of Elaeagnus rhamnoides (L.) A. Nelson in vitro. Biomed. Pharmacother. 2020, 124, 109897. [Google Scholar] [CrossRef]
- Hao, W.; He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Zhao, Y.; Ma, K.Y.; He, W.S.; Chen, Z.Y. Sea buckthorn seed oil reduces blood cholesterol and modulates gut microbiota. Food Funct. 2019, 10, 5669–5681. [Google Scholar] [CrossRef]
- Li, T.S.C.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): A multipurpose plant. HortTechnology 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Bourke, C.D.; Prendergast, C.; Sanin, D.E.; Oulton, T.E.; Hall, R.J.; Mountford, A.P. Epidermal keratinocytes initiate wound healing and pro-inflammatory immune responses following percutaneous schistosome infection. Int. J. Parasitol. 2015, 45, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Ganesh, G.V.; Ramkumar, K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020, 69, 347–363. [Google Scholar] [CrossRef]
- Habbu, P.V.; Joshi, H.; Patil, B.S. Potential wound healers from plant origin. Pharmacogn. Rev. 2007, 1, 271–282. [Google Scholar]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell. Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Jain, U.K. Many of medicinal plants act as wound healers, which has been confirmed in both in vitro and in vivo models. J. Nat. Pharmac. 2010, 1, 2–13. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hormozi, M.; Assaei, R.; Boroujeni, M.B. The effect of Aloe vera on the expression of wound healing factors (TGF-β1 and bFGF) in mouse embryonic fibroblast cell: In vitro study. Biomed. Pharmacother. 2017, 88, 610–616. [Google Scholar] [CrossRef]
- Negahdari, S.; Galehdari, H.; Kesmati, M.; Rezaie, A.; Shariati, G. Wound healing activity of extracts and formulations of Aloe vera, henna, Adiantum capillus-veneris, and myrrh on mouse dermal fibroblast cells. Int. J. Prev. Med. 2017, 8, 18. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Akaki, J.; Yoshida, I.; Matsuoka, N.; Hayakawa, T. Beneficial effects of the genus aloe on wound healing, cell proliferation, and differentiation of epidermal keratinocytes. PLoS ONE 2016, 11, e0164799. [Google Scholar] [CrossRef]
- Atiba, A.; Ueno, H.; Uzuka, Y. The effect of Aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J. Vet. Med. Sci. 2011, 73, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, S.E.; Moridi Farimani, M.; Soroury, S.; Ebrahimi, S.N.; Jabbarzadeh, E. Hypermongone C accelerates wound healing through the modulation of inflammatory factors and promotion of fibroblast migration. Molecules 2019, 24, 2022. [Google Scholar] [CrossRef] [Green Version]
- Nagori, B.P.; Solanki, R. Role of medicinal plants in wound healing. Res. J. Med. Plants. 2011, 5, 392–405. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid. Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żuchowski, J.; Pecio, Ł.; Marciniak, B.; Kontek, R.; Stochmal, A. Unusual isovalerylated flavonoids from the fruit of sea buckthorn (Elaeagnus rhamnoides) grown in Sokółka, Poland. Phytochemistry 2019, 163, 178–186. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Group of Compounds | Relative Peak Area (%) | Dominant Compounds |
|---|---|---|
| phenolic fraction of the fruits (OF) | ||
| Flavonol glycosides | 67.5 | I-3-O-Rut *, I-3-O-Glc *, I-3-O-Glc-7-O-Rha *, Q-3-O-Glc *, rutin *, I-3-O-Glc-7-O-(3′-O-IvA)-Rha |
| Other polar | 21.0 | unidentified, hexose, quinic acid/isomer |
| Triterpenoids and acylated triterpenoids | 9.1 | C30H48O5, C30H48O6 |
| Other non-polar | 2.4 | unidentified |
| non-polar fraction of the fruits (OL) | ||
| Flavonol glycosides | 0.9 | as in the phenolic fraction |
| Other polar | 0.1 | unidentified |
| Triterpenoids and acylated triterpenoids | 83.6 | oleanolic acid *, ursolic acid *, C30H48O5, C30H48O4, C30H48O5-pCouA, C30H48O4-pCouA, C30H48O6-pCouA |
| Other non-polar | 15.4 | unidentified |
| Group of Compounds | Relative Peak Area (%) | Dominant Compounds |
|---|---|---|
| phenolic fraction of the twigs (GF) | ||
| Proanthocyanidins and catechin | 54.3 | catechin, (epi)C-(epi)C, (epi)C-(epi)C-(epi)C, (epi)C-(epi)C-(epi)C-(epi)C, (epi)GC, (epi)C-(epi)GC |
| Flavonol glycosides | 2.3 | I-3-O-Glu-7-O-Rha *, I-3-O-Rut *, K-Hex-CouA |
| Hydrolysable tannins and ellagic acid | 1.9 | ellagic acid, casuarinin/isomer |
| Other polar | 19.8 | dihexose, quinic acid/isomer, unidentified |
| Spermidine derivatives | 10.7 | tricoumaroyl spermidine, feruloyl dicoumaroyl spermidine, diferuloyl coumaroyl spermidine, triferuloyl spermidine |
| Triterpenoids and acylated triterpenoids | 6.7 | C30H48O5, C30H48O4 |
| Other non-polar | 4.3 | unidentified |
| non-polar fraction of the twigs (GL) | ||
| Proanthocyanidins and catechin | 1.3 | catechin, (epi)C-(epi)C, (epi)C-(epi)C-(epi)C |
| Other polar | 1.8 | unidentified |
| Spermidine derivatives | 2.0 | as in the phenolic fraction |
| Triterpenoids and acylated triterpenoids | 89.0 | oleanolic acid *, ursolic acid *, C30H48O5, C30H48O4, C30H48O5-pCouA, C30H48O4-CafA |
| Other non-polar | 5.9 | unidentified |
| Group of Compounds | Relative Peak Area (%) | Dominant Compounds |
|---|---|---|
| phenolic fraction of the leaves (LF) | ||
| Hydrolysable tannins and ellagic acid | 31.3 | casuarinin, casuarictin, strictinin, hippophaenin B or their isomers, ellagic acid |
| Flavonol glycosides | 24.5 | I-3-O-Glc-7-O-Rha *, I-Hex-dHex, I-3-O-Rut *, rutin *, Q-Hex, K-Hex-CouA, I-dHex-Hex-LinA |
| Other polar | 17.4 | dihexose, quinic acid/isomer, (epi)gallocatechin, unidentified |
| Triterpenoid saponins | 15.0 | C71H112O31, C69H110O29, C65H102O27, C65H102O26 |
| Triterpenoids and acylated triterpenoids | 7.6 | C30H48O5, C30H48O4 |
| Other non-polar | 4.2 | unidentified |
| non-polar fraction of the leaves (LL) | ||
| Hydrolysable tannins | 2.7 | casuarinin, hippophaenin B or their isomers |
| Flavonol glycosides | 2.6 | As in the phenolic fraction |
| Other polar | 1.2 | dihexose, quinic acid/isomer, unidentified |
| Triterpenoid saponins | 30.5 | C69H110O29, C63H100O25, C63H100O24, C57H90O20 |
| Triterpenoids and acylated triterpenoids | 50.7 | oleanolic acid *, ursolic acid *, C30H48O5, C30H48O4, C30H48O5-pCouA, C30H48O4-pCouA, C30H48O5-FerA |
| Other non-polar | 12.3 | unidentified |
| Preparation * | Action 6 h | Action 24 h | Control/Significance 6 h; 24 h |
|---|---|---|---|
| OF | ↓ 1.3× | ↓ 1.2× | C1/NS; NS |
| GF | ↓ 2.3× | ↓ 2.1× | C1/p = 0.0138; p = 0.0345 |
| LF | ↓ 3.5× | ↓ 2.0× | C1/p = 0.0138; p = 0.0345 |
| OL | ↓ 2.2× | ↓ 2.9× | C2/p = 0.0138; p = 0.0048 |
| GL | ↓ 1.5× | ↑ 1.3× | C2/p = 0.0138; p = 0.0345 |
| LL | ↓ 1.4× | ↓ 1.2× | C2/p = 0.0138; NS |
| PG | ↑ 1.3× | ↑ 1.7× | C2/p = 0.0138; p = 0.0345 |
| TNF | ↑ 12.5× | ↑ 11.6× | C2/p = 0.0138; p = 0.0345 |
| KU | ↓ 1.2× | ↑ 1.4× | C2/p = 0.0345; p = 0.0345 |
| Q | ↓ 2.7× | ↓ 4.4× | C2/p = 0.0138; p = 0.0345 |
| E | ↓ 1.5× | ↓ 1.3× | C2/p = 0.0138; p = 0.0345 |
| KE | ↓ 2.3× | ↓ 1.1× | C2/p = 0.0138; NS |
| Preparation * | Action 6 h | Action 24 h | Control/Significance 6 h; 24 h |
|---|---|---|---|
| OF | ↑ 2.0× | ↑ 2.0× | C1/p = 0.0138; p = 0.0138 |
| GF | ↓ 1.1× | ↑ 1.8× | C1/p = 0.0138; p = 0.0138 |
| LF | ↑ 1.7× | ↑ 1.5× | C1/NS; NS |
| OL | ↑ 1.7× | ↑ 2.6× | C2/p = 0.0138; p = 0.0133 |
| GL | ↓ 1.2× | ↑ 1.2× | C2/NS; p = 0.0138 |
| LL | ↑ 2.0× | ↑ 1.1× | C2/p = 0.0138; NS |
| PG | ↑ 1.3× | ↑ 1.8× | C2/p = 0.0138; p = 0.0138 |
| TNF | ↑ 4.8× | ↑ 3.0× | C2/p = 0.009; p = 0.0138 |
| KU | ↓ 1.1× | ↑ 3.2× | C2/NS; p = 0.0138 |
| Q | ↓ 3.2× | ↑ 1.7× | C2/p = 0.0138; NS |
| E | no change | ↑ 2.0× | C2/NS; p = 0.0138 |
| KE | ↑ 1.1× | ↑ 2.1× | C2/p = 0.0138; NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, B.; Rywaniak, J.; Cichocka, A.; Cichocka, K.; Żuchowski, J.; Wójcik-Bojek, U.; Więckowska-Szakiel, M.; Różalska, B. Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells. Molecules 2020, 25, 2238. https://doi.org/10.3390/molecules25092238
Sadowska B, Rywaniak J, Cichocka A, Cichocka K, Żuchowski J, Wójcik-Bojek U, Więckowska-Szakiel M, Różalska B. Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells. Molecules. 2020; 25(9):2238. https://doi.org/10.3390/molecules25092238
Chicago/Turabian StyleSadowska, Beata, Joanna Rywaniak, Anna Cichocka, Kinga Cichocka, Jerzy Żuchowski, Urszula Wójcik-Bojek, Marzena Więckowska-Szakiel, and Barbara Różalska. 2020. "Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells" Molecules 25, no. 9: 2238. https://doi.org/10.3390/molecules25092238