Infusions of Herbal Blends as Promising Sources of Phenolic Compounds and Bioactive Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nutritional Analysis and Sugar Composition
2.2. Phenolic Compounds Characterization
2.3. Bioactive Properties
3. Materials and Methods
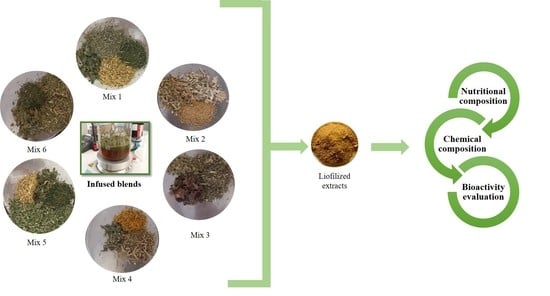
3.1. Samples and Infusions Preparation
3.2. Nutritional Analysis and Sugar Content
3.3. Phenolic Compounds Composition
3.4. Evaluation of Bioactive Properties
3.4.1. Antioxidant Activity
3.4.2. Anti-inflammatory Activity
3.4.3. Cytotoxic Activity in Tumor and Non-Tumor Cells
3.4.4. Antimicrobial Activity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tewari, D.; Samoila, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal plants and natural products used in cataract management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abington, Thames, UK, 2011; ISBN 9781439807132. [Google Scholar]
- Li, S.; Li, S.-K.; Gan, R.-Y.; Song, F.-L.; Kuang, L.; Li, H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Karar, M.G.E.; Kuhnert, N. Herbal Drugs from Sudan: Traditional Uses and Phytoconstituents. Pharmacogn. Rev. 2017, 11, 83–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct. 2016, 7, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Lamiaceae often used in Portuguese folk medicine as a source of powerful antioxidants: Vitamins and phenolics. LWT Food Sci. Technol. 2010, 43, 544–550. [Google Scholar] [CrossRef]
- Guimarães, R.; Calhelha, R.C.; Froufe, H.J.C.; Abreu, R.M.V.; Carvalho, A.M.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Wild Roman chamomile extracts and phenolic compounds: enzymatic assays and molecular modelling studies with VEGFR-2 tyrosine kinase. Food Funct. 2016, 7, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chem. 2013, 136, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Abudunia, A.M.; Marmouzi, I.; Faouzi, M.E.A.; Ramli, Y.; Taoufik, J.; El Madani, N.; Essassi, E.M.; Salama, A.; Khedid, K.; Ansar, M.; et al. Activité anti-candidose, antibactérienne, cytotoxique et antioxydante des fleurs de Calendula arvensis. J. Mycol. Med. 2017, 27, 90–97. [Google Scholar] [CrossRef]
- Dias, M.I.; Barreira, J.C.M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Oliveira, M.B.P.P.; Soković, M.; Ferreira, I.C.F.R. Two-dimensional PCA highlights the differentiated antitumor and antimicrobial activity of methanolic and aqueous extracts of Laurus nobilis L. from different origins. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.G.; Cruz, C.; Faleiro, L.; Simoes, M.T.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Foeniculum vulgare essential oils: chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Commun. 2010, 5, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabeur, I.; Tobaldini, F.; Martins, N.; Barros, L.; Martins, I.; Calhelha, R.C.; Henriques, M.; Silva, S.; Achour, L.; Santos-Buelga, C.; et al. Bioactive properties and functional constituents of Hypericum androsaemum L.: A focus on the phenolic profile. Food Res. Int. 2016, 89, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Putnik, P.; Lorenzo, J.; Barba, F.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Caleja, C.; Finimundy, T.C.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Ivanov, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. A Comparison of the nutritional contribution of thirty-nine aromatic plants used as condiments and/or herbal infusions. Plant Foods Hum. Nutr. 2015, 70, 176–183. [Google Scholar] [CrossRef]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Caracterização do perfil fenólico do extrato aquoso e hidroetanólico de Rosmarinus officinalis L. Rev. Ciências Agrárias 2017, 40, S147–S150. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Nutritional and antioxidant contributions of Laurus nobilis L. leaves: Would be more suitable a wild or a cultivated sample? Food Chem. 2014, 156, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.S.S.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savarese, M.; De Marco, E.; Sacchi, R. Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry. Food Chem. 2007, 105, 761–770. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants:: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Vieira, V.; Pereira, C.; Pires, T.C.S.P.; Calhelha, R.C.; Alves, M.J.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crops Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Soković, M.; Fernández-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C.F.R. Basil as functional and preserving ingredient in “serra da Estrela” cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Santos, P.F. Chemical characterization and bioactive properties of: Geranium molle L.: From the plant to the most active extract and its phytochemicals. Food Funct. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C. Decoction, infusion and hydroalcoholic extract of cultivated thyme: antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, J.; Ho, G.S.; Peng, X.; Ho, C.-T. Isolation and identification of antioxidative flavonoid glycosides from Thyme (Thymus vulgaris L.). J. Food Lipids 1998, 5, 313–321. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD- ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koffi, E.N.; Meudec, E.; Adjé, F.A.; Lozano, P.R.; Lozano, Y.F.; Bekro, Y.-A. Effect of reverse osmosis concentration coupled with drying processes on polyphenols and antioxidant activity obtained from Tectona grandis leaf aqueous extracts. J. Appl. Res. Med. Aromat. Plants 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Neto, R.T.; Silva, A.M.S.; Cardoso, S.M. Health-promoting effects of Thymus herba-barona, Thymus pseudolanuginosus, and Thymus caespititius decoctions. Int. J. Mol. Sci. 2017, 18, 1879. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Wang, L.; Hu, Y.; Zhang, W.; Liu, R. Structural Characterization and Identification of Major Constituents in Jitai Tablets by High-Performance Liquid Chromatography/Diode-Array Detection Coupled with Electrospray Ionization Tandem Mass Spectrometry. Molecules 2012, 17, 10470–10493. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: emerging reserve lots with special harvest requirements. Food Funct. 2016, 7, 4188–4192. [Google Scholar] [CrossRef]
- López-Angulo, G.; Montes-Avila, J.; Díaz-Camacho, S.P.; Vega-Aviña, R.; López-Valenzuela, J.Á.; Delgado-Vargas, F. Comparison of terpene and phenolic profles of three wild species of Echeveria (Crassulaceae). J. Appl. Bot. Food Qual. 2018, 91, 145–154. [Google Scholar] [CrossRef]
- Goncalves, G.A.; Correa, R.C.G.; Barros, L.; Dias, M.I.; Calhelha, R.C.; Correa, V.G.; Bracht, A.; Peralta, R.M.; Ferreira, I. Effects of in vitro gastrointestinal digestion and colonic fermentation on a rosemary (Rosmarinus officinalis L) extract rich in rosmarinic acid. Food Chem. 2019, 271, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Barros, L.; Antonio, A.L.; Ciric, A.; Soković, M.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Foeniculum vulgare Mill. As natural conservation enhancer and health promoter by incorporation in cottage cheese. J. Funct. Foods 2015, 12, 428–438. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barros, L.; Barreira, J.C.M.; Antonio, A.L.; Oliveira, M.B.P.P.; Barreiro, M.F.; Ferreira, I.C.F.R. Cottage cheeses functionalized with fennel and chamomile extracts: Comparative performance between free and microencapsulated forms. Food Chem. 2016, 199, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caleja, C.; Barros, L.; Barreira, J.C.M.; Ciric, A.; Sokovic, M.; Calhelha, R.C.; Beatriz, M.; Oliveira, P.P.; Ferreira, I.C.F.R. Suitability of lemon balm (Melissa officinalis L.) extract rich in rosmarinic acid as a potential enhancer of functional properties in cupcakes. Food Chem. 2018, 250, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Griensven, L.J.L.D. van Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [Green Version]
- Pereira, E.; Antonio, A.L.; Barreira, J.C.M.; Barros, L.; Bento, A.; Ferreira, I.C.F.R. Gamma irradiation as a practical alternative to preserve the chemical and bioactive wholesomeness of widely used aromatic plants. Food Res. Int. 2015, 67, 338–348. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Rockville, MD, USA, 2016. [Google Scholar]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Pinela, J.; Barros, L.; Duenas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012, 135, 1028–1035. [Google Scholar] [CrossRef]
- Svobodova, B.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Walcott, S.; Bittova, M.; Kuban, V.; Ferreira, I.C.F.R. Bioactive properties and phenolic profile of Momordica charantia L. medicinal plant growing wild in Trinidad and Tobago. Ind. Crops Prod. 2017, 95, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Soković, M.; Van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
Sample Availability: Samples are available in dry form the laboratory facilities of CIMO. |
| Mix 1 | Mix 2 | Mix 3 |
 |  |  |
| 50% R. officinalis, 20% C. nobile, 15% L. nobilis, 15% J. regia | 40% F. vulgare, 30% S. nigra, 30% H. perforatum | 50% M. pulegium, 25% O. Europea, 25% V. vinifera |
| Mix 4 | Mix 5 | Mix 6 |
 |  |  |
| 60% M. cervine, 20% C. arvensis 20%, R. idaeus | 60% O. vulgare, 10% C. nobile, 15% L. nobilis, 15% J. regia | 70% T. mastichina, 15% L. nobilis, 15% J. regia |
| Mix 1 | Mix 2 | Mix 3 | Mix 4 | Mix 5 | Mix 6 | |
|---|---|---|---|---|---|---|
| Free sugars (mg/100 mL) | ||||||
| Fructose | 22.1 ± 0.4 b | 13.3 ± 0.6 c | 9.1 ± 0.4 d | 13.3 ± 0.3 c | 30 ± 1 a | nd |
| Glucose | 12.6 ± 0.5 c | 17.1 ± 0.7 a | nd | 3.5 ± 0.1 d | 15.3 ± 0.5 b | nd |
| Sucrose * | nd | nd | nd | nd | 29 ± 1 | 6.5 ± 0.1 |
| Total sugars | 35 ± 1 b | 30 ± 1 c | 9.1 ± 0.4 e | 16.8 ± 0.1 d | 75 ± 2 a | 6.5 ± 0.1 f |
| Energy (cal/100 mL) | 139 ± 4 b | 122 ± 5 c | 36 ± 1 e | 67.2 ± 0.6 d | 298 ± 6 a | 25.8 ± 0.3 f |
| Peak | Rt (min) | λmax (nm) | [M − H] m/z | MS2 | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 4.47 | 328 | 311 | 179(100),149(83),135(72) | Caftaric acid |
| 2 | 4.58 | 324 | 353 | 191(100),179(45),135(7) | 3-O-Caffeoylquinic acid |
| 3 | 5.34 | 319 | 305 | 226(15),175(6),135(40) | Epigallocatechin |
| 4 | 6.45 | 319 | 353 | 191(20),179(50),173(100),135(3) | 4-O-Caffeoylquinic acid |
| 5 | 6.48 | 267 | 305 | 226(13), 225(100),175(2), 97(44) | (+)-Gallocatechin |
| 6 | 6.81 | 319 | 353 | 191(100),179(24),173(37) | 5-O-Caffeoylquinic acid |
| 7 | 7.88 | 322 | 387 | 207(100),163(20) | Tuberonic acid glucoside |
| 8 | 8.8 | 278 | 617 | 287(100) | Eriodictyol-7-O-rutinoside |
| 9 | 9.59 | 320 | 179 | 135(100) | Caffeic acid |
| 10 | 9.8 | 319 | 313 | 197(100) | Salvianolic acid F |
| 11 | 10.74 | 284 | 449 | 287(100) | Eriodictyol-O-hexoside isomer 1 |
| 12 | 11.71 | 340 | 337 | 191(3),173(100),163(43),155(12),119(12) | 4-p-Coumaroylquinic acid |
| 13 | 12.2 | 284 | 449 | 287(100) | Eriodictyol-O-hexoside isomer 2 |
| 14 | 13.33 | 339 | 637 | 285(100) | Luteolin-O-di-glucuronide |
| 15 | 14.25 | 333 | 473 | 311(19),293(19),149(100),135(28) | Chicoric acid |
| 16 | 14.28 | 343 | 537 | 493(7),339(100),295(90) | Salvianolic acid A isomer 1 |
| 17 | 14.4 | 310 | 537 | 493(100),359(33),313(5),295(3) | Lithospermic acid A |
| 18 | 14.72 | 327 | 555 | 537(3),511(3),493(39),311(10),269(20),197(36),179 | Salvianolic acid K |
| 19 | 15.1 | 333 | 521 | 359(50),197(20),179(37),161(100) | Rosmarinic acid hexoside |
| 20 | 15.31 | 334 | 609 | 301(100) | Quercetin-3-O-rutinoside |
| 21 | 15.58 | 344 | 463 | 301(100) | Quercetin-O-hexoside |
| 22 | 15.62 | 335 | 491 | 311(100),293(20),197(12) | Salvianolic acid C |
| 23 | 15.96 | 332 | 797 | 779(100),599(42),555(50),359(37),313(12), 169(5) | Unknown |
| 24 | 16.66 | 351 | 769 | 315(100), 300(10) | Isorhamentin-3-O-rhamnosyl-rutinoside |
| 25 | 17.02 | 332 | 421 | 153(100) | 4-[[(2′,5′Dihydroxybenzoyl)oxy]methyl]phenyl-O-β-d-glucopyranoside |
| 26 | 17.21 | 272, 324sh | 539 | 495(13), 359(21), 297(100), 279(64), 197(34), 179(36), 161(34), 135(18) | Yunnaneic acid D isomer |
| 27 | 17.41 | 308 | 609 | 301(100) | Quercetin-O-rutinoside |
| 28 | 17.9 | 347 | 477 | 301(100) | Quercetin-O-glucuronide |
| 29 | 18.18 | 344 | 717 | 519(100),493(8),339(39),321(92),295(23),197(3) | Salvianolic acid B isomer 1 |
| 30 | 18.45 | 346 | 461 | 285(100) | Luteolin-3’-O-glucuronide |
| 31 | 18.81 | 343 | 447 | 285(100) | Kaempferol-O- hexoside |
| 32 | 19.86 | 337 | 477 | 315(100), 300(19) | Isorhamnetin-3-O-glucoside |
| 33 | 19.9 | 370 | 549 | 505(100),301(74) | Quercetin-7-O-malonylhexoside |
| 34 | 19.97 | 331 | 783 | 513(100), 497(10), 351(3), 289(55), 245(4) | Catechin derivative |
| 35 | 20.24 | 334 | 717 | 519(100),493(8),339(39),321(92),295(23),197(3) | Salvianolic acid B isomer 2 |
| 36 | 20.64 | 343 | 593 | 285(100) | Kaempferol-3-O-rutinoside |
| 37 | 21.56 | 330 | 359 | 197(98), 179(94), 161(100), 135(58) | cis-Rosmarinic acid |
| 38 | 21.65 | 344 | 623 | 315(100),300(10) | Isorhamnetin-3-O-rutinoside |
| 39 | 22.43 | 330 | 719 | 539(12), 521(10), 359(65), 197(6), 179(8), 161(17), 135(3) | Sagerinic acid |
| 40 | 22.54 | 328 | 717 | 519(100), 339(27), 321(87), 295(13), 277(33) | Salvianolic acid L |
| 41 | 22.68 | 325 | 555 | 493(50),292(100),197(36),179(29) | Salvianolic acid K |
| 42 | 22.68 | 330 | 359 | 197(98), 179(94), 161(100), 135(58) | trans-Rosmarinic acid |
| 43 | 23.06 | 337 | 477 | 315(100), 300(19) | Isorhamnetin-3-O-glucoside |
| 44 | 23.69 | 340 | 539 | 377(100), 307(41),275(31) | Oleuropein |
| 45 | 23.75 | 329 | 549 | 505(10), 463(25), 301(100) | Quercetin-O-malonylhexoside |
| 46 | 24.63 | 346 | 461 | 285(100) | Luteolin-3’-O-glucuronide |
| 47 | 25.45 | 331 | 537 | 493(100),359(33),313(5),295(3) | Lithospermic acid A |
| 48 | 25.66 | 344 | 519 | 315(100) | Isorhamnetin-3-O-(6’-acetyl)-glucoside |
| 49 | 28.43 | 327 | 503 | 285(100) | Acetylluteolin-O-glucuronide |
| 50 | 28.89 | 331 | 503 | 285(100) | Acetylluteolin-O-glucuronide |
| 51 | 30.39 | 323 | 493 | 359(84), 313(13), 295(58), 269(7), 197(31), 179(41),161(91),135(86) | Salvianolic acid A isomer 2 |
| 52 | 30.74 | 331 | 503 | 285 (100) | Acetylluteolin-O-glucuronide |
| Peak | Compounds | Mix 1 | Mix 2 | Mix 3 | Mix 4 | Mix 5 | Mix 6 |
|---|---|---|---|---|---|---|---|
| 1 | Caftaric acid | 1.60 ± 0.01 | nd | 1.8 ± 0.2 | nd | nd | nd |
| 2 | 3-O-Caffeoylquinic acid | nd | 3.2 ± 0.2 a | nd | 2.15 ± 0.02 d | 2.56 ± 0.05 c | 2.7 ± 0.1 b |
| 3 | Epigallocatechin | 0.066 ± 0.003 | nd | nd | 1.3 ± 0.1 | nd | nd |
| 4 | 4-O-Caffeoylquinic acid | 0.9 ± 0.1 c | 1.6 ± 0.1 a | nd | nd | nd | 1.2 ± 0.3 b |
| 5 | (+)-Gallocatechin | 2.5 ± 0.2 | nd | nd | nd | nd | nd |
| 6 | 5-O-Caffeoylquinic acid | nd | 22.4 ± 0.2 a | nd | nd | 1.0 ± 0.1 c | 1.6 ± 0.2 b |
| 7 | Tuberonic acid glucoside | 0.216 ± 0.003 | nd | nd | nd | nd | nd |
| 8 | Eriodictyol-7-O-rutinoside | nd | nd | nd | nd | 0.69 ± 0.01 | nd |
| 9 | Caffeic acid | 0.231 ± 0.002 | nd | nd | nd | nd | nd |
| 10 | Salvianolic acid F | nd | nd | nd | 1.50 ± 0.01 | nd | nd |
| 11 | Eriodictyol-O-hexoside isomer 1 | nd | nd | nd | nd | nd | 0.6 ± 0.1 |
| 12 | 4-p-Coumaroylquinic acid | 0.33 ± 0.04 | nd | nd | nd | nd | nd |
| 13 | Eriodictyol-O-hexoside isomer 2 | nd | nd | nd | nd | nd | 3.5 ± 0.6 |
| 14 | Luteolin-O-di-glucuronide | nd | nd | nd | nd | 1.891 ± 0.001 | nd |
| 15 | Chicoric acid | nd | nd | 3.5 ± 0.4 | nd | nd | nd |
| 16 | Salvianolic acid A isomer 1 | nd | nd | nd | nd | 0.48 ± 0.01 | nd |
| 17 | Lithospermic acid A | nd | nd | nd | 1.12 ± 0.03 | nd | nd |
| 18 | Salvianolic acid K | 0.99 ± 0.01 | nd | nd | nd | nd | nd |
| 19 | Rosmarinic acid hexoside | 0.86 ± 0.03 | nd | nd | nd | nd | nd |
| 20 | Quercetin-3-O-rutinoside | nd | 31 ± 1 | nd | 0.20 ± 0.03 | nd | nd |
| 21 | Quercetin-O-hexoside | nd | nd | nd | nd | nd | 8.9 ± 0.1 |
| 22 | Salvianolic acid C | 0.82 ± 0.03 | nd | nd | nd | nd | nd |
| 23 | Unknown | nd | nd | nd | nd | nq | nd |
| 24 | Isorhamentin-3-O-rhamnosyl-rutinoside | nd | nd | nd | 2.51 ± 0.05 | nd | nd |
| 25 | 4-[[(2′,5′Dihydroxybenzoyl)oxy]methyl]phenyl-O-β-d-glucopyranoside | nd | nd | nd | nd | 14.6 ± 0.2 | nd |
| 26 | Yannaneic acid D isomer | nd | nd | nd | 0.6 ± 0.1 | nd | nd |
| 27 | Quercetin-O-rutinoside | nd | nd | 0.62 ± 0.01 | nd | nd | nd |
| 28 | Quercetin-O-glucuronide | nd | nd | 1.98 ± 0.05 | 1.3 ± 0.1 | nd | nd |
| 29 | Salvianolic acid B isomer 1 | nd | nd | 6.3 ± 0.5 | nd | 1.26 ± 0.05 | nd |
| 30 | Luteolin-3’-O-glucuronide | 4.6 ± 0.1 | nd | nd | nd | nd | nd |
| 31 | Kaempferol-O- hexoside | nd | nd | nd | nd | nd | 35 ± 1 |
| 32 | Isorhamnetin-3-O-glucoside | 1.166 ± 0.001 | nd | nd | nd | nd | nd |
| 33 | Quercetin-7-O-malonylhexoside | nd | 1.4 ± 0.2 | nd | nd | nd | nd |
| 34 | Catechin derivative | nd | nd | nd | nd | nq | nd |
| 35 | Salvianolic acid B isomer 2 | nd | nd | nd | 0.95 ± 0.05 | nd | nd |
| 36 | Kaempferol-3-O-rutinoside | nd | 0.6 ± 0.2 | nd | nd | nd | nd |
| 37 | cis-Rosmarinic acid | nd | nd | nd | 13.03 ± 0.05 b | 33.9 ± 0.1 a | 11.826 ± 0.004 c |
| 38 | Isorhamnetin-3-O-rutinoside | nd | 2.7 ± 0.1 | nd | nd | nd | nd |
| 39 | Sagerinic acid | 2.29 ± 0.01 b | nd | 0.93 ± 0.01 c | 0.83 ± 0.05 d | 4.4 ± 0.1 a | nd |
| 40 | Salvianolic acid L | nd | nd | 0.9 ± 0.1 | nd | nd | nd |
| 41 | Salvianolic acid K | nd | nd | nd | nd | nd | 5 ± 1 |
| 42 | trans-Rosmarinic acid | 0.70 ± 0.01 | nd | nd | 0.9 ± 0.1 | nd | nd |
| 43 | Isorhamnetin-3-O-glucoside | nd | 1.08 ± 0.03 | nd | nd | nd | nd |
| 44 | Oleuropein | nd | nd | 9.0 ± 0.3 | nd | nd | nd |
| 45 | Quercetin-O-malonylhexoside | 0.558 ± 0.005 | nd | nd | nd | nd | nd |
| 46 | Luteolin-3’-O-glucuronide | 1.25 ± 0.03 | nd | nd | nd | nd | nd |
| 47 | Lithospermic acid A | 0.6 ± 0.1 c | nd | nd | 0.67 ± 0.03 c | 8.8 ± 0.1 a | 1.91 ± 0.01 b |
| 48 | Isorhamnetin-3-O-(6’-acetyl)-glucoside | nd | 0.90 ± 0.03 | nd | nd | nd | nd |
| 49 | Acetylluteolin-O-glucuronide | 0.57 ± 0.02 | nd | nd | nd | nd | nd |
| 50 | Acetylluteolin-O-glucuronide | 0.65 ± 0.04 | nd | nd | nd | nd | nd |
| 51 | Salvianolic acid A isomer 2 | nd | nd | nd | 4.31 ± 0.05 | nd | nd |
| 52 | Acetylluteolin-O-glucuronide | 0.63 ± 0.04 | nd | nd | nd | nd | nd |
| Total Phenolic Acids | 9.50 ± 0.03 f | 27.2 ± 0.1 b | 22.4 ± 0.1 e | 26.1053 ± 0.0004 c | 67.1 ± 0.2 a | 25 ± 2 d | |
| Total Flavonoids | 12.1 ± 0.1 c | 38 ± 2 b | 3.5 ± 0.1 e | 5.29 ± 0.05 d | 2.58 ± 0.01 f | 47.1 ± 0.2 a | |
| Total Phenolic Compounds | 21.6 ± 0.1 f | 65 ± 2 c | 23.2 ± 0.2 e | 31.40 ± 0.05 d | 69.6 ± 0.2 b | 72 ± 1 a |
| Mix 1 | Mix 2 | Mix 3 | Mix 4 | Mix 5 | Mix 6 | |
|---|---|---|---|---|---|---|
| Antioxidant activity (IC50, µg/mL) | ||||||
| TBARS | 4.5 ± 0.2 f | 23.1 ± 0.3 a | 14 ± 1 c | 22 ± 1 b | 9 ± 1 d | 6.9 ± 0.3 e |
| OxHLIA (Δt = 60 min) | 22 ± 1 c | 106 ± 4 a | 9.7 ± 0.7 e | 12 ± 2 d | 4.0 ± 0.6 f | 35 ± 2 b |
| Cytotoxic activity (GI50, µg/mL) | ||||||
| MCF-7 | 209 ± 5 c | >400 | 238 ± 4 b | >400 | 236 ± 16 b | 254 ± 17 a |
| NCI-H460 | 257 ± 10 b | >400 | 320 ± 12 a | >400 | 250 ± 13 b | 258 ± 16 b |
| HeLa | 246 ± 16 a | >400 | 217 ± 11 c | >400 | 213 ± 9 c | 227 ± 10 b |
| HepG2 | 226 ± 13 b | >400 | 304 ± 6 a | >400 | 230 ± 25 b | 175 ± 18 c |
| PLP2 | >400 | >400 | >400 | >400 | >400 | >400 |
| Anti-inflammatory activity (IC50, µg/mL) | ||||||
| RAW 246.7 | 321 ± 4 a | >400 | 276 ± 12b | >400 | 276 ± 12 b | 262 ± 17 c |
| Mix 1 | Mix 2 | Mix 3 | Mix 4 | Mix 5 | Mix 6 | E221 | E224 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibacterial activity (mg/mL) | ||||||||||||||||
| Bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Gram-negative | ||||||||||||||||
| Salmonella Typhymurium | 0.5 | 1 | 4 | 8 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| Enterobacter cloacae | 0.5 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 4 | 0.5 | 0.5 |
| Gram-positive | ||||||||||||||||
| Bacillus cereus | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 0.5 | 0.5 | 2 | 4 |
| Listeria monocytogenes | 0.5 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0.5 | 1 |
| Staphylococcus aureus | 0.25 | 0.25 | 1 | 1 | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 4 | 4 | 1 | 1 |
| Micrococcus flavus | 2 | 2 | 2 | 4 | 2 | 4 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Antifungal activity (mg/mL) | ||||||||||||||||
| Fungi | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| Aspergillus niger | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | 1 | 1 |
| Aspergillus versicolor | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 1 | 0.25 | 0.5 | 2 | 2 | 1 | 1 |
| Aspergillus fumigatus | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 2 | 0.25 | 5 | 1 | 2 | 1 | 1 |
| Penicillium funiculosum | 0.25 | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.12 | 0.25 | 1 | 2 | 0.5 | 0.5 |
| Penicillium aurantiogriseum | 0.5 | 1 | 1 | 2 | 0.5 | 1 | 1 | 2 | 0.5 | 1 | 0.5 | 1 | 2 | 4 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finimundy, T.C.; Pereira, C.; Dias, M.I.; Caleja, C.; Calhelha, R.C.; Sokovic, M.; Stojković, D.; Carvalho, A.M.; Rosa, E.; Barros, L.; et al. Infusions of Herbal Blends as Promising Sources of Phenolic Compounds and Bioactive Properties. Molecules 2020, 25, 2151. https://doi.org/10.3390/molecules25092151
Finimundy TC, Pereira C, Dias MI, Caleja C, Calhelha RC, Sokovic M, Stojković D, Carvalho AM, Rosa E, Barros L, et al. Infusions of Herbal Blends as Promising Sources of Phenolic Compounds and Bioactive Properties. Molecules. 2020; 25(9):2151. https://doi.org/10.3390/molecules25092151
Chicago/Turabian StyleFinimundy, Tiane C., Carla Pereira, Maria Inês Dias, Cristina Caleja, Ricardo C. Calhelha, Marina Sokovic, Dejan Stojković, Ana Maria Carvalho, Eduardo Rosa, Lillian Barros, and et al. 2020. "Infusions of Herbal Blends as Promising Sources of Phenolic Compounds and Bioactive Properties" Molecules 25, no. 9: 2151. https://doi.org/10.3390/molecules25092151