Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus var. chia from Turkey †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry of Mastic Gum Essential Oils
2.2. Biological Activities of Mastic Gum Essential Oils
3. Materials and Methods
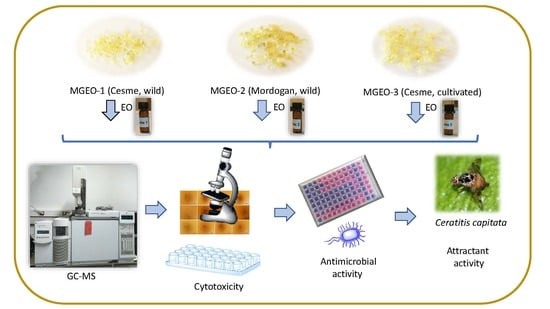
3.1. Plant Material and Essential Oil Isolation
3.2. Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC-MS)
3.3. Chiral Analysis of α-Pinene
3.4. Cell Lines and Maintenance
3.5. Cytotoxicity Assay
3.6. Morphological Studies
3.7. Inhibition of iNOS Activity
3.8. In Vitro Antimicrobial Evaluation
3.8.1. Microbial Strains and Culture Media
3.8.2. Microdilution Assay
3.9. Short-Range Attraction Bioassays with Ceratitis Capitata
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Browicz, K. Pistacia lentiscus cv. chia (Anacardiaceae) on Chios island. Plant Syst. Evol. 1987, 155, 189–195. [Google Scholar] [CrossRef]
- Kokolakis, A.K.; Kouyarakis, A.N.; Katerinopoulos, H.E. Effect of hydrodistillation with phosphoric acid on the yield of Chios mastic gum essential oil. Flavour Fragr. J. 2010, 25, 48–53. [Google Scholar] [CrossRef]
- Dimas, K.S.; Pantazis, P.; Ramanujam, R. Review: Chios mastic gum: A plant-produced resin exhibiting. numerous diverse pharmaceutical and biomedical properties. In Vivo 2012, 26, 777–785. [Google Scholar] [PubMed]
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, L.A.; Aligiannis, N. Supercritical CO2 extraction of mastic gum and chemical characterization of bioactive fractions using LC-HRMS/MS and GC–MS. J. Supercrit. Fluids 2018, 133, 349–356. [Google Scholar] [CrossRef]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorgi, N. Pistacia genus as a potential source of neuroprotective natural products. Planta Med. 2019, 85, 1326–1350. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- El Bishbishy, M.H.; Gad, H.A.; Nora, M.; Aborehab, N.M. Chemometric discrimination of three Pistacia species via their metabolic profiling and their possible in vitro effects on memory functions. J. Pharm. Biomed. Anal. 2020, 177, 112840. [Google Scholar] [CrossRef]
- The European Medicines Agency (EMA); Committee on Herbal Medicinal Products (HMPC). Assessment Report on Pistacia Lentiscus L., Resin (Mastix). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2015/07/WC500190097.pdf (accessed on 7 February 2019).
- Triantafyllou, A.; Chaviaras, N.; Sergentanis, T.N.; Protopapa, E.; Tsaknis, J. Chios mastic gum modulates serum biochemical parameters in a human population. J. Ethnopharmacol. 2007, 111, 43–49. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Kiosseoglou, V. Chios Mastic Gum and Its Food Applications. In Functional Properties of Traditional Foods; Kristbergsson, K., Otles, S., Eds.; Springer: New York, NY, USA, 2016; pp. 271–287. [Google Scholar]
- Giaginis, C.; Theocharis, S. Current evidence on the anticancer potential of Chios mastic gum. Nutr. Cancer 2011, 63, 1174–1184. [Google Scholar] [CrossRef]
- Van den Berg, K.J.; van der Horst, J.; Boon, J.J.; Sudeijer, O.O. Cis-1,4-poly-β-myrcene; the structure of the polymeric fraction of mastic resin (Pistacia lentiscus L.) elucidated. Tetrahedron Lett. 1998, 39, 2645–2648. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Goulas, V.; Gekas, V. Health-promoting effects of Pistacia resins: Recent advances, challenges, and potential applications in the food industry. Food Rev. Int. 2015, 31, 1–12. [Google Scholar] [CrossRef]
- Aksoy, A.; Duran, N.; Koksal, F. In vitro and in vivo antimicrobial effects of mastic chewing gum against Streptococcus mutans and mutans streptococci. Arch. Oral Biol. 2006, 51, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Duran, N.; Toroglu, S.; Koksal, F. Short-term effect of mastic gum on salivary concentrations of cariogenic bacteria in orthodontic patients. Angle Orthod. 2007, 77, 124–128. [Google Scholar] [CrossRef]
- Freedman, P. Mastic: A Mediterranean luxury product. Mediterr. Hist. Rev. 2011, 26, 99–113. [Google Scholar] [CrossRef]
- Greek Gastronomy Guide. Available online: http://www.greekgastronomyguide.gr/en/item/mastiha-chios-mastic-gum-growers-association/ (accessed on 7 February 2019).
- Kahve Dunyasi. Available online: https://www.kahvedunyasi.com/kahve-c-1798 (accessed on 17 February 2019).
- Yavuzer, R.; Kelly, C.; Durrani, N.; Mittal, V.; Jackson, I.T.; Remine, S. Reinforcement of subcuticular continuous suture closure with surgical adhesive strips and gum mastic: Is there any additional strength provided? Am. J. Surg. 2005, 189, 315–318. [Google Scholar] [CrossRef]
- Know-how of cultivating mastic on the island of Chios. Available online: https://ich.unesco.org/en/RL/know-how-of-cultivating-mastic-on-the-island-of-chios-00993 (accessed on 7 February 2019).
- European Pharmacopoeia, 10th ed.; Monograph 1876; Council of Europe: Strasbourg, France, 2019.
- Kivcak, B.; Akay, B.; Demirci, B.; Baser, K.H.C. Chemical composition of essential oils from leaves and twigs of Pistacia lentiscus, Pistacia lentiscus var. chia, and Pistacia terebinthus from Turkey. Pharm. Biol. 2004, 42, 360–366. [Google Scholar] [CrossRef]
- Ozden, S. The economic analysis of the mastic tree (Pistacia lentiscus L.) cultivation projects. Anatol. J. For. Res. 2019, 5, 93–100. [Google Scholar]
- Turkiye Bitkileri Listesi (Damarli Bitkiler); Nezahat Gokyigit Botanik Bahcesi ve Flora Arastirmalari Dernegi Yayini, Istanbul: Istanbul, Turkey, 2012; Available online: https://bizimbitkiler.org.tr/yeni/demos/technical/ (accessed on 19 April 2020).
- Koc, I.; Onay, A.; Ciftci, Y.O. In vitro regeneration and conservation of the lentisk (Pistacia lentiscus L.). Turk. J. Biol. 2014, 38, 653–663. [Google Scholar] [CrossRef]
- TEMA, Turkey Combating Soil Erosion, for Reforestation and the Protection of Natural Resources Foundation. Available online: http://www.tema.org.tr/web_14966-2_1/index.aspx (accessed on 19 April 2020).
- Parlak, S. Clonal propagation of mastic tree (Pistacia lentiscus var. chia Duham.) in outdoor beds using different rootstock and grafting techniques. J. For. Res. 2018, 29, 1061–1067. [Google Scholar] [CrossRef]
- Tabanca, N.; United States Department of Agriculture, Agricultural Research Service, Subtropical Horticulture Research Station (SHRS), Miami, FL, USA; Nalbantsoy, A.; Department of Bioengineering, Faculty of Engineering, Ege University, Bornova, Izmir, Turkey. Personal communication, 8 July 2018.
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com/database/kovats/kovats-detailsulcatone.php (accessed on 17 March 2019).
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/f_kovats.html (accessed on 25 April 2020).
- Boelens, M.H.; Jimenez, R. Chemical composition of the essential oils from the gum and various parts of Pistacia lentiscus L. (mastic gum tree). Flavour Fragr. J. 1991, 6, 271–275. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Mellidis, A.S. The chemical composition of the essential oil of mastic gum. J. Essent. Oil Res. 1991, 3, 107–110. [Google Scholar] [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Daferea, D.; Pappas, C.; Tarantilis, P.A.; Polissiou, M. Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. 2002, 77, 511–515. [Google Scholar] [CrossRef]
- Duru, M.E.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Martin Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. Chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Gortzi, O.; Athanasiadis, V.; Lalas, S.; Chinou, I.; Tsaknis, J. Study of antioxidant and antimicrobial activity of Chios mastic gum fractions (neutral, acidic) before and after encapsulation in liposomes. J. Food Process. Technol. 2014, 5, 1. [Google Scholar]
- Paraschos, S.; Magiatis, P.; Gikas, E.; Smyrnioudis, I.; Skaltsounis, A.L. Quality profile determination of Chios mastic gum essential oil and detection of adulteration in mastic oil products with the application of chiral and non-chiral GC–MS analysis. Fitoterapia 2016, 114, 12–17. [Google Scholar] [CrossRef]
- Rigling, M.; Fraatz, M.A.; Stefan Troegel, S.; Jinyuan Sun, J.; Holger Zorn, H.; Zhang, Y. Aroma investigation of Chios mastic gum (Pistacia lentiscus variety chia) using headspace gas chromatography combined with olfactory detection and chiral analysis. J. Agric. Food Chem. 2019, 67, 13420–13429. [Google Scholar] [CrossRef]
- Serifi, I.; Tzima, E.; Bardouki, H.; Lampri, E.; Papamarcaki, T. Effects of the essential oil from Pistacia lentiscus var. chia on the lateral line system and the gene expression profile of zebrafish (Danio rerio). Molecules 2019, 24, 3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogan, Y.; Baslar, S.; Aydin, H.; Mert, H.H. A study of the soil-plant interactions of Pistacia lentiscus L. distributed in the western Anatolian part of Turkey. Acta Bot. Croat. 2003, 62, 73–88. [Google Scholar]
- Papanicolaou, D.; Melanitou, M.; Katsaboxakis, K. Changes in chemical composition of the essential oil of Chios “mastic resin” from Pistacia lentiscus var. Chia tree during solidification and storage. Dev. Food Sci. 1995, 37, 303–310. [Google Scholar]
- Zhou, L.; Satoh, K.; Takahashi, K.; Watanabe, S.; Nakamura, W.; Maki, J.; Hatano, H.; Takekawa, F.; Shimada, C.; Sakagami, H. Re-evaluation of anti-inflammatory activity of mastic using activated macrophages. In Vivo 2009, 23, 583–599. [Google Scholar]
- Bouyahya, A.; Assemian, I.C.C.; Mouzount, H.; Bourais, I.; Et-Touys, A.; Fellah, H.; Benjouad, A.; Dakka, N.; Bakri, Y. Could volatile compounds from leaves and fruits of Pistacia lentiscus constitute a novel source of anticancer, antioxidant, antiparasitic and antibacterial drugs? Ind. Crops Prod. 2019, 218, 62–69. [Google Scholar] [CrossRef]
- Balan, K.V.; Prince, J.; Han, Z.; Dimas, K.; Cladaras, M.; Wyche, J.H.; Sitaras, N.M.; Pantazis, P. Antiproliferative activity and induction of apoptosis in human colon cancer cells treated in vitro with constituents of a product derived from Pistacia lentiscus L. var. chia. Phytomedicine 2007, 14, 263–272. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 3782. [Google Scholar] [CrossRef] [Green Version]
- Magkouta, S.; Stathopoulos, G.T.; Psallidas, I.; Papapetropoulos, A.; Kolisis, F.N.; Roussos, C.; Loutrari, H. Protective effects of mastic oil from Pistacia lentiscus variation chia against experimental growth of lewis lung carcinoma. Nutr. Cancer 2009, 61, 640–648. [Google Scholar] [CrossRef]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. Intermediacy of H2O2- and p53-dependent pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef] [Green Version]
- Attoub, S.; Karam, S.M.; Nemmar, A.; Arafat, K.; John, A.; Al-Dhaheri, W.; Al Sultan, M.A.; Raza, H. Short-term effects of oral administration of Pistacia lentiscus oil on tissue-specific toxicity and drug metabolizing enzymes in mice. Cell Physiol Biochem. 2014, 33, 1400–1410. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Sakagami, H.; Amano, S.; Fukuchi, K.; Sunaga, K.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Shirataki, Y.; Tomomura, M.; et al. Evaluation of biological activity of mastic extracts based on chemotherapeutic indices. In Vivo 2017, 31, 591–598. [Google Scholar]
- Kaliora, A.C.; Stathopoulou, G.M.; Triantafillidis, J.K.; Dedoussis, G.V.Z.; Andrikopoulos, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Morkhade, D.M. Evaluation of gum mastic (Pistacia lentiscus) as a microencapsulating and matrix forming material for sustained drug release. Asian J. Pharm. Sci. 2017, 12, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Fukazawa, M.; Motohira, H.; Ochiai, K.; Nishikawa, H.; Miyata, T. A pilot study in anti-plaque effects of mastic chewing gum in oral cavity. J. Periodontol. 2003, 74, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT-Food Sci. Technol. 2018, 97, 109–110. [Google Scholar] [CrossRef]
- Jang, E.B.; Douglas, I.; Light, M.; Flath, R.A.; Nagata, J.T.; Mon, T.R. Electroantennogram responses of the Mediterranean fruit fly, Ceratitis capitata to identified volatile constituents from calling males. Entomol. Exp. Appl. 1989, 50, 7–19. [Google Scholar] [CrossRef]
- Light, D.M.; Jang, E.B.; Flath, R.A. Electroantennogram responses of the Mediterranean fruit fly, Ceratitis capitata, to the volatile constituents of nectarines. Entomol. Exp. Appl. 1992, 63, 13–26. [Google Scholar] [CrossRef]
- Casana-Giner, V.; Gandia-Balaguer, A.; Hernandez-Alamos, M.M.; Mengod-Puerta, C.; Garrido-Vivas, A.; Primo-Millo, J.; Primo-Yufera, E. Attractiveness of 79 compounds and mixtures to wild Ceratitis capitata (Diptera: Tephritidae) in field trials. J. Econ. Entomol. 2001, 94, 898–904. [Google Scholar] [CrossRef]
- Niogret, J.; Gill, M.A.; Espinoza, H.R.; Kendra, P.E. Attraction and electroantennogram responses of male Mediterranean fruit fly (Diptera: Tephritidae) to six plant essential oils. J. Entomol. Zool. Stud. 2017, 5, 958–964. [Google Scholar]
- European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2004; Volume 1.
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Joulain, D.; Koenig, W.A.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils; Library of MassFinder 3: Hamburg, Germany, 2004. [Google Scholar]
- Joulain, D.; Koenig, W.A. The Atlas of Spectra Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- ESO 2000. The Complete Database of Essential Oils. Boelens Aroma Chemical Information Service; BACIS: Huizen, The Netherlands, 1999. [Google Scholar]
- Surakka, A.; Sihvonen, M.L.; Lehtimaki, M.J.; Wahlsten, M.; Vuorela, P.; Sivonen, K. Benthic cyanobacteria from the Baltic Sea contain cytotoxic Anabaena, Nodularia and Nostoc strains and an apoptosis inducing Phormidium strain. Environ. Toxicol. 2005, 20, 285–292. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast Approved Standard, M27-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, CLSI M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 17th Informational Supplement, M100-S17, 27:1; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- Niogret, J.; Montgomery, W.S.; Kendra, P.E.; Heath, R.R.; Epsky, N.D. Attraction and electroantennogram responses of male Mediterranean fruit fly to volatile chemicals from Persea, Litchi and Ficus wood. J. Chem. Ecol. 2011, 37, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Masi, M.; Epsky, N.; Nocera, P.; Cimmino, A.; Kendra, P.E.; Niogret, J.; Evidente, A. Laboratory evaluation of natural and synthetic aromatic compounds as potential attractants for male Mediterranean fruit fly, Ceratitis capitata. Molecules 2019, 24, 2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epsky, N.D.; Niogret, J. Short range attraction of Ceratitis capitata (Diptera: Tephritidae) sterile males to six commercially available plant essential oils. Nat. Volatiles Essent. Oils 2017, 4, 1–7. [Google Scholar]
- Systat Software. SigmaPlot for Windows v. 14.0; Systat Software, Inc.: San Jose, CA, USA, 2017. [Google Scholar]
Sample Availability: Samples of essential oils are not available. |




| RRI a | RRI b | Compound | MGEO-1 (Wild) % | MGEO-2 (Wild) % | MGEO-3 (Cultivated) % | IM * |
|---|---|---|---|---|---|---|
| 1014 | 1012c | Tricyclene | - | - | 0.6 | MS |
| 1032 | 1008–1039 c | α-Pinene | 56.2 | 51.9 | 70.8 | RRI, MS |
| 1076 | 1043–1086 c | Camphene | 0.2 | 0.2 | 2.3 | RRI, MS |
| 1118 | 1085–1130 c | β-Pinene | 2.7 | 3.1 | 5.7 | RRI, MS |
| 1132 | 1098–1140 c | Sabinene | 0.4 | 0.5 | 0.6 | RRI, MS |
| 1135 | 1109–1137 c | Thuja-2,4(10)-diene | tr | tr | 0.1 | MS |
| 1174 | 1140–1175 c | Myrcene | 20.1 | 18.6 | 2.5 | RRI, MS |
| 1203 | 1212 d | Limonene | 1.7 | 2.4 | 2.3 | RRI, MS |
| 1218 | 1188–1233 c | β-Phellandrene | tr | tr | - | RRI, MS |
| 1280 | 1246–1291 c | p-Cymene | 0.1 | 0.2 | 0.4 | RRI, MS |
| 1348 | 1317–1357 c | 6-Methyl-5-hepten-2-one | tr | tr | tr | MS |
| 1384 | 1331–1384 c | α-Pinene oxide | 0.5 | 0.6 | 0.4 | RRI, MS |
| 1417 | 4,8-Dimethyl-1,3,7-nonatriene | 0.3 | 0.3 | tr | MS | |
| 1424 | o-Methyl anisol | 0.7 | 0.5 | 0.6 | RRI, MS | |
| 1429 | 1405–1431 c | Perillene | 1.9 | 2.2 | 1.5 | MS |
| 1466 | 1438–1480 c | α-Cubebene | tr | - | tr | MS |
| 1497 | 1462–1522 c | α-Copaene | 0.3 | 0.1 | 0.3 | RRI, MS |
| 1499 | α-Campholene aldehyde | 0.1 | tr | 0.1 | MS | |
| 1535 | 1496–1546 c | β-Bourbonene | 0.3 | 0.1 | 0.4 | MS |
| 1549 | 1518–1560 c | β-Cubebene | 0.1 | - | 0.1 | MS |
| 1553 | 1507–1564 c | Linalool | 0.4 | 0.3 | 0.4 | RRI, MS |
| 1565 | 1532–1570 c | Linalyl acetate | 0.4 | 0.2 | 0.2 | RRI, MS |
| 1586 | 1545–1590 c | Pinocarvone | 0.2 | 0.2 | 0.2 | RRI, MS |
| 1591 | 1549–1597 c | Bornyl acetate | 0.2 | - | 0.7 | RRI, MS |
| 1600 | 1565–1608 c | β-Elemene | tr | - | 0.1 | RRI, MS |
| 1612 | 1569–1632 c | β-Caryophyllene | 3.3 | 6.8 | 2.8 | RRI, MS |
| 1648 | 1597–1648 c | Myrtenal | 0.2 | 0.2 | 0.2 | MS |
| 1661 | 1624–1668 c | Alloaromadendrene | 0.2 | - | 0.1 | MS |
| 1670 | 1643–1671 c | trans-Pinocarveol | 0.2 | 0.3 | 0.4 | RRI, MS |
| 1683 | 1665–1691 c | trans-Verbenol | 0.7 | 0.6 | 0.4 | MS |
| 1687 | 1663 e | α-Humulene | 0.7 | 0.9 | 0.5 | RRI, MS |
| 1704 | 1689 c 1681 e | γ-Muurolene | 0.4 | tr | 0.2 | MS |
| 1706 | 1694 c 1688 e | α-Terpineol | 0.1 | 0.1 | 0.2 | RRI, MS |
| 1709 | 1685–1709 c | α-Terpinyl acetate | 0.3 | 0.2 | 0.2 | RRI, MS |
| 1725 | 1696–1735 c | Verbenone | 0.1 | 0.2 | 0.2 | RRI, MS |
| 1733 | 1693–1740 c | Neryl acetate | 0.1 | 0.2 | 0.1 | RRI, MS |
| 1740 | 1686–1753 c | α-Muurolene | 0.2 | - | 0.2 | MS |
| 1772 | (Z)-Anethole | tr | 0.1 | tr | RRI, MS | |
| 1773 | 1755 c 1749 e | δ-Cadinene | 0.2 | - | 0.2 | MS |
| 1804 | 1743–1808 c | Myrtenol | 0.2 | 0.1 | 0.2 | MS |
| 1845 | 1802–1846 c | (E)-Anethole | 0.3 | 0.2 | tr | RRI, MS |
| 1849 | Calamenene | 0.1 | - | tr | MS | |
| 1849 | 1766–1849 c | Cuparene | 0.1 | 0.1 | - | MS |
| 1864 | 1813–1865 c | p-Cymen-8-ol | 0.1 | 0.1 | 0.2 | RRI, MS |
| 1900 | 1854–1928 c | epi-Cubebol | 0.1 | - | tr | MS |
| 1912 | p-Cymen-9-ol | 0.1 | 0.1 | 0.1 | MS | |
| 1957 | 1884–1964 c | Cubebo | 0.2 | - | 0.1 | MS |
| 2001 | Isocaryophyllene oxide | 0.4 | 0.8 | 0.1 | MS | |
| 2008 | 1936–2023 e | Caryophyllene oxide | 2.1 | 4.1 | 0.9 | RRI, MS |
| 2029 | 1963–2029 e | Perilla alcohol | 0.1 | 0.1 | 0.1 | RRI, MS |
| 2053 | 1980–2050 e | Anisaldehyde | - | 0.1 | - | RRI, MS |
| 2071 | 2003–2071 e | Humulene epoxide-I | 0.3 | 0.4 | 0.1 | MS |
| 2109 | cis-Methyl isoeugenol | 0.1 | 0.1 | 0.2 | MS | |
| 2144 | Dimyrcene Ia | 0.1 | 0.1 | tr | MS | |
| 2174 | Dimyrcene Ib | 0.1 | 0.1 | 0.1 | MS | |
| 2200 | trans-Methyl isoeugenol | 0.1 | tr | 0.1 | MS | |
| 2219 | Dimyrcene IIa | 0.9 | 1.1 | 0.5 | RRI, MS | |
| 2269 | Dimyrcene IIb | 0.3 | 0.4 | 0.2 | RRI, MS | |
| 2380 | 8α,13-Oxy-14-en-epilabdane (=epi-Manoyl oxide) | - | - | 0.2 | MS | |
| 2392 | Caryophylla-2(12),6-dien-5β-ol (=Caryophyllenol II) | - | 0.1 | - | MS | |
| 2415 | 3,4-Dimethoxy benzaldehyde | tr | - | tr | MS | |
| Monoterpene Hydrocarbons | 81.4 | 76.9 | 85.3 | |||
| Oxygenated Monoterpenes | 6.2 | 6.0 | 5.8 | |||
| Sesquiterpene Hydrocarbons | 5.9 | 8.0 | 4.9 | |||
| Oxygenated Sesquiterpenes | 3.1 | 5.4 | 1.2 | |||
| Diterpenes | 1.4 | 1.7 | 1.0 | |||
| Others | 1.2 | 1.0 | 0.9 | |||
| Total | 99.2 | 99.0 | 99.1 |
| Origin | Main Components (%) | Extraction Methods | Ref. |
|---|---|---|---|
| Spain | α-pinene (78.6), β-pinene (3.3), myrcene (3.2) | Hex | [33] |
| Greece | α-pinene (58.9, 77.1), myrcene (0.2, 12.3), linalool (3.7, 0.5) | SD | [34] |
| Commercial | α-pinene (66.5), myrcene (8.3), β-pinene (3.3) | SD | [35] |
| Commercial | α-pinene (33.7–72.8), myrcene (3.8–63.5) | - | [36] |
| Chios Island | α-pinene (72.1), myrcene (16.5), β-pinene (2.9) | SDE | |
| Turkey (Fethiye) | β-pinene (38.7), α-pinene (21.7), pinocarvone (5.3), limonene (3.8), n-nonanal (3.5) | D | [37] |
| Commercial | α-pinene (63.3), myrcene (25), β-pinene (3.3) | - | [38] |
| CMGGA | α-pinene (40.9), (Z,Z)-farnesol (11.9), β-caryophyllene (5.3), myrcene (9.0), β-pinene (1.7) | EtOH soluble part | |
| Greece | α-pinene (25.6), verbenone (14), p-cymene (9.8), verbenene (8.6), 2-methylanisole (6.5), 1,2-dimethyl-4-ethyl benzene (6), pinocarvone (4.9), myrtenal (3) | SPME | [39] |
| CMGGA | α-pinene (59.2–87.1), myrcene (4.7–27.6), β-pinene (1.6–3.6), β-caryophyllene (0.1–4.9) | - | [40] |
| Greece | α-pinene (67.5), β-pinene (2.8), verbenone (2.6), trans-pinocarveol (2.5), p-mentha-1,5-dien-8-ol (2.4), myrcene (1.1) | HD | [5] |
| α-pinene (34.9-46), verbenone (3.2–5.5), trans-verbenol (5.9–7.1), caryophyllene oxide (2.5–3.9), β-caryophyllene (1.8–3.8), trans-pinocarveol (1.6–2.2), β-pinene (1.4–2.1) | SFE | ||
| Chios Island | α-pinene, β-pinene, camphene, myrcene, 2-nonanone, perillene, linalool, terpinen-4-ol | SPME-SBSE | [41] |
| CMGGA | α-pinene (67.7), myrcene (18.8), β-pinene (3.1) | DD | [42] |
| Experimental Results Mean ± SD * (n = 3) | (−)/(+)- α-Pinene | Myrcene/(+)- α-Pinene | (−)-α-Pinene/ Myrcene | Myrcene/ α-Pinene | β-Pinene/(+)- α-Pinene | β-Pinene/ Myrcene |
|---|---|---|---|---|---|---|
| MGEO-1 (wild) | 0.0058 ± 0.0009 | 0.20 ± 0 | 0.029 ± 0.004 | 0.36 | 0.027 ± 0 | 0.029 ± 0.004 |
| MGEO-2 (wild) | 0.0051 ± 0.0005 | 0.19 ± 0.009 | 0.027 ± 0.002 | 0.36 | 0.031 ± 0 | 0.074 ± 0.002 |
| MGEO-3 (cultivated) | 0.0060 ± 0.0007 | 0.025 ± 0 | 0.24 ± 0.028 | 0.04 | 0.023 ± 0 | 0.24 ± 0.028 |
| Literature Values [40] | ||||||
| 45 Authentic Chios MGEOs (min-max) | 0.0055–0.010 | 0.06–0.34 | 0.019–0.11 | 0.06–0.34 | 0.020–0.038 | |
| Average Chios MGEOs | 0.0071 ± 0014 | 0.18 ± 0.06 | 0.045 ± 0018 | 0.027 ± 0.0062 | ||
| 19 Commercial products | 0–0.67 | 0–0.74 | ||||
| Cell Lines | IC50 μg/mL | |||
|---|---|---|---|---|
| MGEO-1 (Wild) | MGEO-2 (Wild) | MGEO-3 (Cultivated) | Doxorubicin * | |
| PANC-1 | 14.76 ± 2.68 | 18.05 ± 3.72 | 46.87 ± 3.70 | 26.48 ± 2.16 |
| MCF-7 | 47.45 ± 1.45 | 39.52 ± 6.85 | 38.69 ± 4.33 | 20.25 ± 0.41 |
| MDA-MB-231 | 23.08 ± 0.55 | 26.55 ± 0.16 | 12.40 ± 0.39 | 19.33 ± 0.50 |
| PC3 | 15.3 ± 0.29 | 30.67 ± 5.72 | 19.54 ± 0.09 | 6.35 ± 0.38 |
| CaCo-2 | 31.74 ± 5.84 | 49.91 ± 0.40 | 47.60 ± 3.72 | 10.25 ± 0.26 |
| HeLa | 20.11 ± 4.15 | 18.81 ± 0.73 | 7.621 ± 1.91 | 2.14 ± 0.26 |
| 253J-BV | 15.96 ± 1.08 | 10.82 ± 1.25 | 12.14 ± 1.93 | 2.50 ± 1.30 |
| A549 | 7.02 ± 1.94 | 5.77 ± 1.65 | 19.93 ± 1.23 | 5.57 ± 1.09 |
| RAW264.7 | 7.62 ± 0.51 | 9.20 ± 2.64 | 5.84 ± 0.68 | 1.23 ± 0.53 |
| SK-MEL-30 | 5.25 ± 0.33 | 5.25 ± 0.81 | 13.31 ± 2.53 | 2.09 ± 0.18 |
| U87MG | 11.71 ± 2.74 | 6.12 ± 1.49 | 23.42 ± 3.21 | 6.38 ± 1.05 |
| HepG2 | 25.19 ± 2.51 | 44.83 ± 1.42 | 47.47 ± 2.54 | 5.63 ± 0.07 |
| HEK293 | 15.74 ± 0.08 | 17.83 ± 3.00 | 17.66 ± 1.58 | 1.19 ± 0.17 |
| Concentration (μg/mL) | MGEO-1 (Wild) | MGEO-2 (Wild) | MGEO-3 (Cultivated) | Doxorubicin * |
|---|---|---|---|---|
| 50 | 105.83 ± 3.15 | 106.79 ± 0.89 | 105.19 ± 1.35 | |
| 5 | 11.35 ± 0.89 | 17.39 ± 1.35 | 6.89 ± 0.89 | |
| 0.5 | 15.48 ± 11.25 | 7.53 ± 3.59 | 10.07 ± 6.29 | |
| 20 | 94.06 ± 2.65 | |||
| 2 | 6.47 ± 1.23 | |||
| 0.2 | 4.55 ± 0.89 |
| Samples | IC50 (μg/mL) |
|---|---|
| MGEO-1 (wild) | 11.6 ± 0.9 |
| MGEO-2 (wild) | 13.75 ± 0.4 |
| MGEO-3 (cultivated) | 13.85 ± 0.5 |
| Doxorubicin * | 7.2 ± 0.1 |
| Samples | E. coli NRRL B-3008 | S. aureus ATCC 6538 | S. typhimurium ATCC 13311 | L. monocytogenes ATCC 19111 | K. pneumoniae NCTC 9633 | C. albicans ATCC 90028 |
|---|---|---|---|---|---|---|
| MGEO-1 (wild) | >10 | 5 | 2.5 | 5 | 5 | 1.25 |
| MGEO-2 (wild) | >10 | >10 | >10 | >10 | 5 | 2.5 |
| MGEO-3 (cultivated) | >10 | >10 | 5 | >10 | >10 | 2.5 |
| Chloramphenicol ** | 8 | 8 | 8 | 4 | 8 | - |
| Amoxicillin ** | 0.25 | >32 | <0.062 | <0.062 | <0.062 | |
| Amphotericin B ** | - | - | - | - | - | 0.125 |
© 2020 This work was produced by US government employees and is in the public domain in the US. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus var. chia from Turkey. Molecules 2020, 25, 2136. https://doi.org/10.3390/molecules25092136
Tabanca N, Nalbantsoy A, Kendra PE, Demirci F, Demirci B. Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus var. chia from Turkey. Molecules. 2020; 25(9):2136. https://doi.org/10.3390/molecules25092136
Chicago/Turabian StyleTabanca, Nurhayat, Ayse Nalbantsoy, Paul E. Kendra, Fatih Demirci, and Betul Demirci. 2020. "Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus var. chia from Turkey" Molecules 25, no. 9: 2136. https://doi.org/10.3390/molecules25092136