Fourier Transform Infrared Spectroscopy Based Complementary Diagnosis Tool for Autism Spectrum Disorder in Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Stage
2.1.1. Patients and Control Group Selection
2.1.2. Samples Preparation
2.2. Spectroscopic Stage
2.2.1. Sample Measurements
2.2.2. Data Pre-processing
2.2.3. Classification Models Development and Testing
3. Results and Discussion
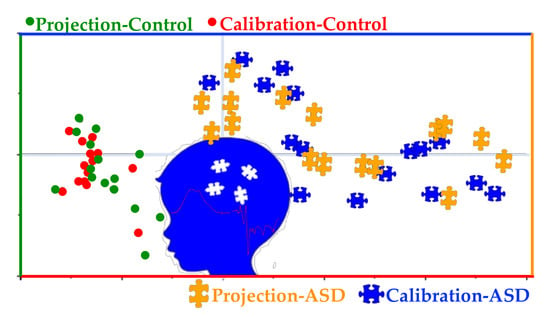
3.1. Preliminary Data Analysis
3.2. Classification Models Development
3.3. Predictions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zurawicz, E.; Kaluzna-Czaplinska, J. Analysis of amino acids in autism spectrum disorders. Trends Anal. Chem. 2015, 73, 91–118. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Autism and Development Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators and Centers for Disease Control and Prevention. MMWR Surveill. Summ. 2012, 61, 1–19.
- Ruggeri, B.; Sarkans, U.; Schumann, G.M.; Persico, A. Biomarkers in autism spectrum disorder: The old and the new. Psychopharmacology 2014, 231, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Wetie, A.G.N.; Wormwood, K.; Thome, J.; Dudley, E.; Taurines, R.; Gerlach, M.; Woods, A.G.; Darie, C.C. A Pilot Proteomic Study of Protein Markers in Autism Spectrum Disorder. Electrophoresis 2014, 35, 2046–2054. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Ghezzo, A.; Bolotta, A.; Ferreri, C.; Minguzzi, R.; Vignini, A.; Visconti, P.; Marini, M. Perspective Biological Markers for Autism Spectrum Disorders: Advantages of the Use of Receiver Operating Characteristic Curve s in Evaluating Marker Sensitivity and Specificity. Dis. Markers 2015, 329607, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Eikeseth, S.; Klintwall, L.; Jahr, L.E.; Karlsson, P. Outcome for children with autism receiving early and intensive behavioral intervention in mainstream preschool and kindergarten settings. Res. Autism Spectr. Disord. 2012, 6, 829–835. [Google Scholar] [CrossRef]
- Eldevik, S.; Hastings, P.R.; Hughes, C.J.; Jahr, E.; Eikeseth, S.; Cross, S. Meta-Analysis of Early Intensive Behavioral Intervention for Children With Autism. J. Clin. Child. Adolesc. Psych. 2009, 38, 439–450. [Google Scholar] [CrossRef]
- Goldani, A.A.S.; Downs, S.R.; Widjaja, F.; Lawton, B.; Hendren, R.L. Biomarkers in autism. Front. Psychiatry 2014, 5, 1–13. [Google Scholar] [CrossRef]
- ElBaz, F.M.; Zaki, M.M.; Youssef, A.M.; ElDorry, G.F.; Elalfy, D.Y. Study of plasma amino acid levels in children with autism: An Egyptian sample. Egypt. J. Med. Hum. Genet. 2014, 15, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Tezdig, I.; Tarakcioglu, M.C.; Kadak, M.T.; Demirel, O.F.; Ozer, O.F. Serum levels of glial fibrillary acidic protein and Nogo-A in children with autism spectrum disorders. Biomarkers 2016, 21, 614–618. [Google Scholar] [CrossRef]
- West, P.R.; Amaral, D.G.; Bais, P.; Smith, A.M.; Egnash, L.A.; Ross, M.E.; Palmer, J.A.; Fontaine, B.R.; Conard, K.R.; Corbett, B.A.; et al. Metabolomics as a Tool for Discovery of Biomarkers of Autism Spectrum Disorder in the Blood Plasma of Children. PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, J.; Zhang, J.; Li, K. Serum levels of SOD and risk of autism spectrum disorder: A case-control study. Int. J. Devl. Neurosci. 2016, 51, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Jory, J. Abnormal fatty acids in Canadian children with autism. Nutrition 2016, 32, 474–477. [Google Scholar] [CrossRef]
- Kondolota, M.; Ozmert, E.N.; Asci, A.; Erkekoglu, P.; Oztop, D.B.; Gumus, H.; Kocer-Gumus, B.; Yurdak, K. Plasma phthalate and bisphenol a levels and oxidant-antioxidant status in autistic children. Env. Toxicol. Pharm. 2016, 43, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Smaga, I.; Niedzielska, E.; Gawlik, M.; Moniciewski, A.; Krzek, J.; Przegalinski, E.; Pera, J.; Filip, M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharm. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Yazdani, U.; Deng, Y.; Li, W.; Gadad, B.S.; Hynan, L.; Karp, D.; Roatch, N.; Schutte, C.; Marti, C.N.; et al. Search for Blood Biomarkers for Autism: Peptoids. Sci. Rep. 2016, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kanagathara, N.; Thirunavukkarasu, M.; Jeyanthi, E.C.; Shenbagarajan, P. FTIR and UV-Visible spectral study on normal blood samples. Int. J. Pharm. Bio. Sci. 2011, 1, 74–81. [Google Scholar]
- Ahmed, S.S.S.J.; Santosh, W.; Kumar, S.; Christlet, T.H.T. Neural network algorithm for the early detection of Parkinson’s disease from blood plasma by FTIR micro-spectroscopy. Vib. Spectrosc. 2010, 53, 181–188. [Google Scholar] [CrossRef]
- Conti, C.; Giorgini, E.; Pieramici, T.; Rubini, C.; Tosi, G. FT-IR microscopy imaging on oral cavity tumours, II. J. Mol. Struct. 2005, 744, 187–193. [Google Scholar] [CrossRef]
- Deleris, G.; Petibois, C. Applications of FT-IR spectrometry to plasma contents analysis and monitoring. Vib. Spectrosc. 2003, 32, 129–136. [Google Scholar] [CrossRef]
- Perez-Guaita, D.; Sanchez-lllana, A.; Ventura-Gayete, J.; Garrigue, S.; Guardia, M. Chemometric determination of lipidic parameters in serum using ATR measurements of dry films of solvent extracts. Analyst 2014, 139, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, M.; Ghasemi, K.; Garmarudi, A.B.; Ramin, M. Diagnostic prediction of renal failure from blood serum analysis by FTIR spectrometry and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Dovbeshko, G.I.; Gridina, N.Y.; Kruglova, E.B.; Pashchuk, O.P. FTIR spectroscopy studies of nucleic acid damage. Talanta 2000, 53, 233–246. [Google Scholar] [CrossRef]
- Mostaco-Guidolin, L.B.; Bachmann, L. Application of FTIR Spectroscopy for Identification of Blood and Leukemia Biomarkers: A Review over the Past 15 Years. Appl. Spectrosc. Rev. 2011, 46, 388–404. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Talyshinsky, M.; Souprun, Y.; Huleihel, M. FTIR spectroscopy examination of leukemia patients plasma. Vibrat. Spectrosc. 2006, 40, 40–46. [Google Scholar] [CrossRef]
- Lewis, P.D.; Lewis, K.E.; Ghosal, R.; Bayliss, S.; Lloyd, A.J.; Wills, J.; Godfrey, R.; Kloer, P.; Mur, L.A.J. Evaluation of FTIR Spectroscopy as a diagnostic tool for lung cancer using sputum. BMC Cancer 2010, 10, 640. [Google Scholar] [CrossRef] [Green Version]
- Mordechai, S.; Shufan, E.; Katz Porat, B.S.; Salman, A. Early diagnosis of Alzheimer’s disease using infrared spectroscopy of isolated blood samples followed by multivariate analyses. Analyst 2017, 142, 1276–1284. [Google Scholar] [CrossRef]
- Gassaloglu, S.I.; Baykara, B.; Avcil, S.; . Demiral, Y. Validity and Reliability Analysis of Turkish Version of Childhood Autism Rating Scale. Turk Psikiyatr. Derg. 2016, 27, 266–274. [Google Scholar]
- Wold, H. Quantitative Sociology: International Perspectives on Mathematical and Statistical Model Building; Academic Press: New York, NY, USA, 1975; pp. 307–357. [Google Scholar]
- CAMO Software Inc. The Unscrambler Version 10.5; CAMO A/S: Trondheim, Norway, 2018. [Google Scholar]
- Barker, M.; Rayens, W. Partial Least Squares For Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Ward, J.H.J. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Wiest, M.M.; German, J.B.; Harvey, D.J.; Watkins, S.M.; Hertz-Picciotto, I. Plasma fatty acid profiles in autism: A case-control study. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.E.; Souders, M.C.; Ittenbach, R.F.; Giarelli, E.; Mulberg, A.E.; Pinto-Martin, J.A. Relationship of dietary intake to gastrointestinal symptoms in children with autistic spectrum disorders. Biol. Psychiatry 2007, 61, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar]
- Liu, K.Z.; Shi, M.H.; Mantsch, H.H. Molecular and chemical characterization of blood cells by infrared spectroscopy: A new optical tool in hematology. Blood Cells Mol. Dis. 2005, 35, 404–412. [Google Scholar] [CrossRef]
- Krilov, D.; Balarin, M.; Kosovic, M.; Gamulin, O.; Brnjas-Kralkevic, J. FT-IR spectroscopy of lipoproteins-a comparative study. Spectrochim. Acta A 2009, 73, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Petibois, C.; Rigalleau, V.; Melin, A.M.; Perromat, A.; Cazorla, G.; Gin, H.; Deleris, G. Determination of glucose in dried serum samples by Fourier-transform infrared spectroscopy. Clin. Chem. 1999, 45, 1530–1535. [Google Scholar] [CrossRef] [Green Version]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Snowden, J.S.; Saxon, J.A.; Richardson, A.M.T.; Jones, M.; Mann, D.M.A.; Allsop, D.; Martin-Hirsch, P.L.; et al. Differential diagnosis of Alzheimer’s disease using spectrochemical analysis of blood. Proc. Natl. Acad. Sci. USA 2017, 2017, E7929–E7938. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, P.; Reddy, P.; Kumar, K.V.; Candu, B.R.; Rajendran, K. Evaluation of metformin hydrochloride in Wistar rats by FTIR-ATR spectroscopy: A convenient tool in the clinical study of diabetes. J. Nat. Sci. Biol. Med. 2014, 5, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Sheng, D.; Liu, X.; Li, W.; Yang, Y.; Chen, X.; Wang, X. Distinction of leukemia patients’ and healthy persons’ serum using FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 228–232. [Google Scholar] [CrossRef]
- Croonenberghs, J.; Bosmans, E.; Deboutte, D.; Kenis, G.; Maes, M. Activation of the Inflammatory Response System in Autism. Neuropsychobiology 2002, 45, 1–6. [Google Scholar] [CrossRef]
- Tu, W.J.; Chen, H.; He, J. Application of LC-MS/M analysis of plasma amino acids profiles in children with Autism. J. Clin. Biochem. Nutr. 2012, 5, 1248–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirouvanziam, R.; Obukhanych, T.V.; Laval, J.; Aronov, P.A.; Libove, R.; Banerjee, A.G.; Parker, K.J.; Ohara, R.; Herzenberg, L.A.; Herzenberg, L.A.; et al. Distinct Plasma Profile of Polar Neutral Amino Acids, Leucine, and Glutamate in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 42, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Mei, H.; Pan, X.C.; Tan, W.; Liu, T.F.; Yang, L. An improved large-scale prediction model of CYP1A2 inhibitors by using combined fragment descriptors. Chemom. Intell. Lab. Syst. 2014, 130, 109–114. [Google Scholar] [CrossRef]
- Campos, N.D.S.; Oliveira, K.S.; Almeida, M.R.; Stephan, R.; Oliveira, L.F.C. Classification of Frankfurters by FT-Raman Spectroscopy and Chemometric Methods. Molecules 2014, 19, 18980–18992. [Google Scholar] [CrossRef] [Green Version]
- De Vries, S.; Ter Braak, C.J.F. Prediction error in partial least squares regression: A critique on the deviation used in The Unscrambler. Chemom. Intell. Lab. Syst. 1995, 30, 239–245. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| ASD | Age | Sex | ASD | Age | Sex | Control | Age | Sex | Control | Age | Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 10 | B | A16 | 9 | B | C1 | 13 | B | C16 | 10 | B |
| A2 | 9 | B | A17 | 7 | B | C2 | 8 | G | C17 | 11 | B |
| A3 | 6 | B | A18 | 4 | G | C3 | 16 | B | C18 | 10 | B |
| A4 | 7 | G | A19 | 8 | B | C4 | 9 | B | C19 | 8 | B |
| A5 | 12 | B | A20 | 5 | B | C5 | 7 | G | C20 | 8 | B |
| A6 | 14 | B | A21 | 5 | B | C6 | 11 | B | C21 | 16 | B |
| A7 | 4 | G | A22 | 5 | B | C7 | 12 | B | C22 | 10 | B |
| A8 | 10 | B | A23 | 7 | B | C8 | 9 | G | C23 | 10 | B |
| A9 | 14 | G | A24 | 17 | B | C9 | 6 | G | C24 | 11 | B |
| A10 | 5 | B | A25 | 7 | B | C10 | 14 | B | C25 | 8 | B |
| A11 | 7 | B | A26 | 7 | B | C11 | 9 | G | C26 | 12 | B |
| A12 | 6 | B | A27 | 13 | G | C12 | 13 | G | C27 | 8 | G |
| A13 | 17 | B | A28 | 8 | G | C13 | 16 | B | C28 | 8 | B |
| A14 | 14 | B | A29 | 4 | B | C14 | 12 | B | C29 | 10 | B |
| A15 | 5 | B | A30 | 13 | G | C15 | 8 | B | C30 | 7 | G |
 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogruc Ildiz, G.; Bayari, S.; Karadag, A.; Kaygisiz, E.; Fausto, R. Fourier Transform Infrared Spectroscopy Based Complementary Diagnosis Tool for Autism Spectrum Disorder in Children and Adolescents. Molecules 2020, 25, 2079. https://doi.org/10.3390/molecules25092079
Ogruc Ildiz G, Bayari S, Karadag A, Kaygisiz E, Fausto R. Fourier Transform Infrared Spectroscopy Based Complementary Diagnosis Tool for Autism Spectrum Disorder in Children and Adolescents. Molecules. 2020; 25(9):2079. https://doi.org/10.3390/molecules25092079
Chicago/Turabian StyleOgruc Ildiz, Gulce, Sevgi Bayari, Ahmet Karadag, Ersin Kaygisiz, and Rui Fausto. 2020. "Fourier Transform Infrared Spectroscopy Based Complementary Diagnosis Tool for Autism Spectrum Disorder in Children and Adolescents" Molecules 25, no. 9: 2079. https://doi.org/10.3390/molecules25092079