Structure-Dependent Activity of Plant-Derived Sweeteners
Abstract
:1. Introduction
2. Method
3. Plant-Derived Sweeteners
3.1. Carbohydrates
3.1.1. Sugars
3.1.2. Sugar Alcohols
3.2. Amino Acids
3.3. Phenols
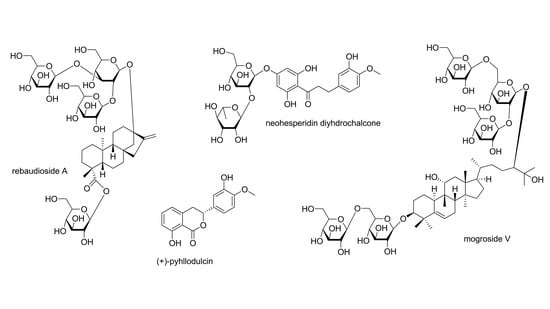
3.3.1. Phyllodulcin and Derivatives
3.3.2. Flavanonols
3.3.3. Dihydrochalcones
3.3.4. Condensed Phenols
3.4. Monoterpenes
3.5. Sesquiterpenes
3.6. Diterpenes
3.6.1. Kauran-Type Diterpenoids
3.6.2. Lanostan- and Gibban-Type Diterpenoids
3.7. Triterpenes
3.7.1. Oleanan-Type Triterpenoids
3.7.2. Cycloartan- and Dammaran-Type Triterpenoids
3.7.3. Cucurbitane-Type Triterpenoids
3.7.4. Steroids
4. Conclusions
Funding
Conflicts of Interest
References
- Munger, S.D.; Meyerhof, W. The Molecular Basis of Gustatory Transduction. In Handbook of Olfaction and Gustation; Wiley: Hoboken, NJ, USA, 2015; pp. 685–700. [Google Scholar]
- Behrens, M.; Meyerhof, W. G Protein–Coupled Taste Receptors; Elsevier BV: Amsterdam, Netherlands, 2016; pp. 227–244. [Google Scholar]
- DeSimone, J.A.; Dubois, G.E.; Lyall, V. Chemical Modulators of Taste. In Handbook of Olfaction and Gustation; Wiley: Hoboken, NJ, USA, 2015; pp. 665–684. [Google Scholar]
- Spaggiari, G.; Di Pizio, A.; Cozzini, P. Sweet, umami and bitter taste receptors: State of the art of in silico molecular modeling approaches. Trends Food Sci. Technol. 2020, 96, 21–29. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The Receptors for Mammalian Sweet and Umami Taste. Cell 2003, 115, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Nuemket, N.; Yasui, N.; Kusakabe, Y.; Nomura, Y.; Atsumi, N.; Akiyama, S.; Nango, E.; Kato, Y.; Kaneko, M.K.; Takagi, J.; et al. Structural basis for perception of diverse chemical substances by T1r taste receptors. Nat. Commun. 2017, 8, 15530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temussi, P. The Sweet Taste Receptor: A Single Receptor with Multiple Sites and Modes of Interaction. In Advances in Food and Nutrition Research; Elsevier BV: Amsterdam, Netherlands, 2007; Volume 53, pp. 199–239. [Google Scholar]
- Sharma, V.K.; Ingle, N.A.; Kaur, N.; Yadav, P.; Ingle, E.; Charania, Z. Sugar Substitutes and Health: A Review. J. Adv. Oral Res. 2016, 7, 7–11. [Google Scholar] [CrossRef]
- Chéron, J.-B.; Golebiowski, J.; Antonczak, S.; Fiorucci, S. The anatomy of mammalian sweet taste receptors. Proteins: Struct. Funct. Bioinform. 2017, 85, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Dubois, G.E.; Prakash, I. Non-Caloric Sweeteners, Sweetness Modulators, and Sweetener Enhancers. Annu. Rev. Food Sci. Technol. 2012, 3, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Kinghora, A.D.; Soejarto, D.D.; Inglett, G.E. Sweetening agents of plant origin*. Crit. Rev. Plant Sci. 1986, 4, 79–120. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and Umami Taste: Natural Products, Their Chemosensory Targets, and Beyond. Angew. Chem. Int. Ed. 2011, 50, 2220–2242. [Google Scholar] [CrossRef]
- Bassoli, A.; Merlini, L.; Morini, G. Isovanillyl sweeteners. From molecules to receptors. Pure Appl. Chem. 2002, 74, 1181–1187. [Google Scholar] [CrossRef]
- Çiçek, S.S.; Vitalini, S.; Zidorn, C. Natural Phenyldihydroisocoumarins: Sources, Chemistry and Bioactivity. Nat. Prod. Commun. 2018, 13, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Shallenberger, R.S.; Acree, T.E. Molecular Theory of Sweet Taste. Nature 1967, 216, 480–482. [Google Scholar] [CrossRef]
- Kier, L.B. A Molecular Theory of Sweet Taste. J. Pharm. Sci. 1972, 61, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Nofre, C. New hypotheses for the GPCR 3D arrangement based on a molecular model of the human sweet-taste receptor. Eur. J. Med. Chem. 2001, 36, 101–108. [Google Scholar] [CrossRef]
- Dubois, G.E. Molecular mechanism of sweetness sensation. Physiol. Behav. 2016, 164, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, C.; Peña, I.; Mata, S.; Alonso, J.L. Sweet Structural Signatures Unveiled in Ketohexoses. Chem. Eur. J. 2016, 22, 16829–16837. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.R.; León, I.; Kolesniková, L.; Alonso, J.L. The Structural Signs of Sweetness in Artificial Sweeteners: A Rotational Study of Sorbitol and Dulcitol. ChemPhysChem 2018, 19, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.R.; León, I.; Kolesnikova, L.; Alonso, J.L. Rotational Spectrum of Saccharin: Structure and Sweetness. J. Phys. Chem. A 2019, 123, 2756–2761. [Google Scholar] [CrossRef]
- Leach, K.; Gregory, K.J.; Leach, K. Molecular insights into allosteric modulation of Class C G protein-coupled receptors. Pharmacol. Res. 2017, 116, 105–118. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Staszewski, L.; Tang, H.; Adler, E.; Zoller, M.; Li, X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 14258–14263. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Ji, Q.; Liu, Z.; Benard, L.M.J.; Margolskee, R.F.; Max, M.; Snyder, L.A. The Cysteine-rich Region of T1R3 Determines Responses to Intensely Sweet Proteins. J. Boil. Chem. 2004, 279, 45068–45075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-K.; Chen, Y.; Abrol, R.; Goddard, W.A.; Guthrie, B. Activation mechanism of the G protein-coupled sweet receptor heterodimer with sweeteners and allosteric agonists. Proc. Natl. Acad. Sci. USA 2017, 114, 2568–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 31 March 2020).
- Eteraf-Oskouei, T.; Najafi, M.; Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar]
- Rose, D.J.; Anderson, J.J. Sweeteners in our society. World Rev. Nutr. Diet. 1983, 41, 200–231. [Google Scholar]
- Inglett, G. Sweeteners - a Review. Food Technol. 1981, 35, 37. [Google Scholar]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Shankar, P.; Ahuja, S.; Sriram, K. Non-nutritive sweeteners: Review and update. Nutrition 2013, 29, 1293–1299. [Google Scholar] [CrossRef]
- Grembecka, M. Sugar alcohols – their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda Alters Gut Microflora and Increases Intestinal P-Glycoprotein and Cytochrome P-450 in Male Rats. J. Toxicol. Environ. Health Part A 2008, 71, 1415–1429. [Google Scholar] [CrossRef]
- Qin, X. What made Canada become a country with the highest incidence of inflammatory bowel disease: Could sucralose be the culprit? Can. J. Gastroenterol. 2011, 25, 511. [Google Scholar] [CrossRef]
- O’Brien-Nabors, L. Alternative Sweeteners, Fourth Edition; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-4614-8. [Google Scholar]
- Elbein, A.D.; Pan, Y.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Vigues, S.; Hobbs, J.R.; Conn, G.L.; Munger, S.D. Distinct Contributions of T1R2 and T1R3 Taste Receptor Subunits to the Detection of Sweet Stimuli. Curr. Boil. 2005, 15, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Servant, G.; Tachdjian, C.; Tang, X.-Q.; Werner, S.; Zhang, F.; Li, X.; Kamdar, P.; Petrovic, G.; Ditschun, T.; Java, A.; et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl. Acad. Sci. USA 2010, 107, 4746–4751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, K.; Koizumi, A.; Nakajima, K.-I.; Tanaka, T.; Abe, K.; Misaka, T.; Ishiguro, M. Characterization of the Modes of Binding between Human Sweet Taste Receptor and Low-Molecular-Weight Sweet Compounds. PLoS ONE 2012, 7, e35380. [Google Scholar] [CrossRef] [PubMed]
- Solms, J.; Vuataz, L.; Egli, R.H. The taste ofl- andd-amino acids. Cell. Mol. Life Sci. 1965, 21, 692–694. [Google Scholar] [CrossRef]
- Mazur, R.H.; Schlatter, J.M.; Goldkamp, A.H. Structure-taste relationships of some dipeptides. J. Am. Chem. Soc. 1969, 91, 2684–2691. [Google Scholar] [CrossRef]
- Iwamura, H. Structure--sweetness relationship of L-aspartyl dipeptide analogues. A receptor site topology. J. Med. Chem. 1981, 24, 575–583. [Google Scholar] [CrossRef]
- Vleggaar, R.; Ackerman, L.G.J.; Steyn, P.S. Structure elucidation of monatin, a high-intensity sweetener isolated from the plant Schlerochiton ilicifolius. J. Chem. Soc. Perkin Trans. 1992, 1, 3095–3098. [Google Scholar] [CrossRef]
- Bassoli, A.; Borgonovo, G.; Busnelli, G.; Morini, G.; Merlini, L. Monatin, Its Stereoisomers and Derivatives: Modeling the Sweet Taste Chemoreception Mechanism. Eur. J. Org. Chem. 2005, 2005, 2518–2525. [Google Scholar] [CrossRef]
- Rousseau, A.L.; Buddoo, S.R.; Gordon, G.E.R.; Beemadu, S.; Kupi, B.G.; Lepuru, M.J.; Maumela, M.C.; Parsoo, A.; Sibiya, D.M.; Brady, D. Scale-Up of a Chemo-Biocatalytic Route to (2R,4R)- and (2S,4S)-Monatin. Org. Process. Res. Dev. 2011, 15, 249–257. [Google Scholar] [CrossRef]
- Bassoli, A.; Borgonovo, G.; Morini, G. Lost and found in sweeteners: forgotten molecules and unsolved problems in the chemistry of sweet compounds. Flavour Fragr. J. 2011, 26, 269–273. [Google Scholar] [CrossRef]
- Bassoli, A.; Drew, M.G.B.; Hattotuwagama, C.K.; Merlini, L.; Morini, G.; Wilden, G.R. Quantitative Structure-Activity Relationships of Sweet Isovanillyl Derivatives. Quant. Struct. Relationships 2001, 20, 3–16. [Google Scholar] [CrossRef]
- Winnig, M.; Bufe, B.; Kratochwil, N.A.; Slack, J.P.; Meyerhof, W. The binding site for neohesperidin dihydrochalcone at the human sweet taste receptor. BMC Struct. Boil. 2007, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Nanayakkara, N.P.D.; Hussain, R.A.; Pezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D. Potential sweetening agents of plant origin. 13. An intensely sweet dihydroflavonol derivative based on a natural product lead compound. J. Med. Chem. 1988, 31, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, H.; Mabry, T.J.; Kinghorn, A.D. Dihydroflavonol sweeteners and other constituents from Hymenoxys turneri. Phytochemistry 1990, 29, 2865–2869. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamasaki, K.; Kohda, H.; Tanaka, O.; Mahato, S. Dihydrochalcone-glucosides as Sweet Principles of Symplocos ssp. Planta Medica 1980, 40, 81–83. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawamura, K.; Kohda, H.; Yamasaki, K.; Tanaka, O. Glycosides of the leaves of Symplocos spp. (Symplocaceae). Chem. Pharm. Bull. 1982, 30, 2421–2423. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, R.M.; Gentili, B. Taste and structure in phenolic glycosides. J. Agric. Food Chem. 1969, 17, 696–700. [Google Scholar] [CrossRef]
- Inglett, G.E.; Krbechek, L.; Dowling, B.; Wagner, R. Dihydrochalcone Sweeteners? Sensory and Stability Evaluation. J. Food Sci. 1969, 34, 101–104. [Google Scholar] [CrossRef]
- Dubois, G.E.; Crosby, G.A.; Stephenson, R.A.; Wingard, R.E. Dihydrochalcone sweeteners. Synthesis and sensory evaluation of sulfonate derivatives. J. Agric. Food Chem. 1977, 25, 763–772. [Google Scholar] [CrossRef]
- Whitelaw, M.L.; Daniel, J.R. Synthesis and sensory evaluation of ring-substituted dihydrochalcone sweeteners. J. Agric. Food Chem. 1991, 39, 44–51. [Google Scholar] [CrossRef]
- Arnoldi, A.; Bassoli, A.; Borgonovo, G.; Merlini, L. Synthesis and sweet taste of optically active (–)-haematoxylin and of some (±)-haematoxylin derivatives. J. Chem. Soc. Perkin Trans. 1995, 1, 2447–2453. [Google Scholar] [CrossRef]
- Baek, N.-I.; Chung, M.-S.; Shamon, L.; Kardono, L.B.S.; Tsauri, S.; Padmawinata, K.; Pezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D. Selligueain A, a Novel Highly Sweet Proanthocyanidin from the Rhizomes of Selliguea feei. J. Nat. Prod. 1993, 56, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Acton, E.M.; Stone, H.; Leaffer, M.A.; Oliver, S.M. Perillartine and some derivatives: Clarification of structures. Cell. Mol. Life Sci. 1970, 26, 473–474. [Google Scholar] [CrossRef]
- Acton, E.; Stone, H. Potential new artificial sweetener from study of structure-taste relationships. Science 1976, 193, 584–586. [Google Scholar] [CrossRef]
- Compadre, C.; Pezzuto, J.; Kinghorn, A.D.; Kamath, S. Hernandulcin: an intensely sweet compound discovered by review of ancient literature. Science 1985, 227, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, A.D.; Kaneda, N.; Baek, N.-I.; Kennelly, E.J.; Soejarto, D.D. Noncariogenic intense natural sweeteners. Med. Res. Rev. 1998, 18, 347–360. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Soejarto, D.D. Discovery of terpenoid and phenolic sweeteners from plants. Pure Appl. Chem. 2002, 74, 1169–1179. [Google Scholar] [CrossRef]
- Briedel, M.; Lavielle, R. Sur le principe sucré des feuilles de Kaá-êh-é (Stevia rebaudiana B). C. R. Acad. Sci. Paris 1931, 192, 1123–1125. [Google Scholar]
- Crammer, B.; Ikan, R. Properties and syntheses of sweetening agents. Chem. Soc. Rev. 1977, 6, 431. [Google Scholar] [CrossRef]
- Geuns, J.M. Stevioside. Phytochemistry 2003, 64, 913–921. [Google Scholar] [CrossRef]
- Soejarto, D.; Addo, E.M.; Kinghorn, A.D. Highly sweet compounds of plant origin: From ethnobotanical observations to wide utilization. J. Ethnopharmacol. 2019, 243, 112056. [Google Scholar] [CrossRef] [PubMed]
- Makapugay, H.; Nanayakkara, N.; Kinghorn, A.D. Improved high-performance liquid chromatographic separation of the Stevia rebaudiana sweet diterpene glycosides using linear gradient elution. J. Chromatogr. A 1984, 283, 390–395. [Google Scholar] [CrossRef]
- Prakash, I.; Dubois, G.; Clos, J.; Wilkens, K.; Fosdick, L. Development of rebiana, a natural, non-caloric sweetener. Food Chem. Toxicol. 2008, 46, S75–S82. [Google Scholar] [CrossRef] [PubMed]
- DuBois, G.E.; Stephenson, R.A. Diterpenoid sweeteners. Synthesis and sensory evaluation of stevioside analogs with improved organoleptic properties. J. Med. Chem. 1985, 28, 93–98. [Google Scholar] [CrossRef]
- Acevedo, W.; Ramírez-Sarmiento, C.A.; Agosin, E. Identifying the interactions between natural, non-caloric sweeteners and the human sweet receptor by molecular docking. Food Chem. 2018, 264, 164–171. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohda, H.; Tanaka, O.; Chen, F.-H.; Chou, W.-H.; Leu, J.-L. Rubusoside (β- d -Glucosyl Ester of 13- O -β- d -Glucosyl-steviol), a Sweet Principle of Rubus chingii Hu (Rosaceae). Agric. Boil. Chem. 1981, 45, 2165–2166. [Google Scholar] [CrossRef]
- Ohtani, K.; Aikawa, Y.; Kasai, R.; Chou, W.-H.; Yamasaki, K.; Tanaka, O. Minor diterpene glycosides from sweet leaves of Rubus suavissimus. Phytochemistry 1992, 31, 1553–1559. [Google Scholar] [CrossRef]
- Darise, M.; Mizutani, K.; Kasai, R.; Tanaka, O.; Kitahata, S.; Okada, S.; Ogawa, S.; Murakami, F.; Chen, F.-H. Enzymic transglucosylation of rubusoside and the structure-sweetness relationship of steviol-bisglycosides. Agric. Biol. Chem. 1984, 48, 2483–2488. [Google Scholar]
- Tahara, A.; Nakata, T.; Ohtsuka, Y. Biological Sciences: New Type of Compound with Strong Sweetness. Nature 1971, 233, 619–620. [Google Scholar] [CrossRef]
- Kim, N.-C.; Kinghorn, A.D. Highly sweet compounds of plant origin. Arch. Pharmacal Res. 2002, 25, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Esaki, S.; Konishi, F.; Kamiya, S. Synthesis and taste of some glycosides of glycyrrhetic acid. Agric. Biol. Chem. 1978, 42, 1599–1600. [Google Scholar]
- Brieskorn, C.H.; Lang, J. 18 β-Glycyrrhetinsäure und süßer Geschmack. Arch. der Pharm. 1978, 311, 1001–1009. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Nakano, K.; Pongpiriyadacha, Y.; Murakami, T.; Matsuda, H. Characterization of New Sweet Triterpene Saponins fromAlbizia myriophylla1. J. Nat. Prod. 2002, 65, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Ishizone, H.; Suganuma, M.; Ogura, M.; Nakatsu, K.; Yoshioka, H. Periandrin I, a sweet triterpene-glycoside from periandra dulcis. Phytochemitry 1983, 22, 259–264. [Google Scholar] [CrossRef]
- Suttisri, R.; Chung, M.-S.; Kinghorn, A.D.; Sticher, O.; Hashimoto, Y. Periandrin V, a further sweet triterpene glycoside from Periandra dulcis. Phytochemistry 1993, 34, 405–408. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Kinghorn, A.D.; Shi, X.; Zhang, H.; Teo, B.K. Abrusoside A: a new type of highly sweet triterpene glycoside. J. Chem. Soc., Chem. Commun. 1989, 887. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Hussain, R.A.; Pezzuto, J.M.; Kinghorn, A.D.; Morton, J.F. Abrusosides A-D, Four Novel Sweet-Tasting Triterpene Glycosides from the leaves of Abrus precatorius. J. Nat. Prod. 1989, 52, 1118–1127. [Google Scholar] [CrossRef]
- Kennelly, E.J.; Cai, L.; Kim, N.-C.; Kinghorn, A.D. Abrusoside e, a further sweet-tasting cycloartane glycoside from the leaves of Abrus precatorius. Phytochemistry 1996, 41, 1381–1383. [Google Scholar] [CrossRef]
- Yang, D.J.; Zhong, Z.C.; Xie, Z.M. Studies on the sweet principles from the leaves of Cyclocarya paliurus (Batal.) Iljinskaya. Acta Pharm. Sin. 1992, 27, 841–844. [Google Scholar]
- Shu, R.G.; Xu, C.R.; Li, L.N. Studies on the sweet principles from the leaves of Cyclocarya paliurus (Batal.) Iljinsk. Acta Pharm. Sin. 1995, 30, 757–761. [Google Scholar]
- Kennelly, E.J.; Cai, L.; Long, L.; Shamon, L.; Zaw, K.; Zhou, B.-N.; Pezzuto, J.M.; Kinghorn, A.D. Novel Highly Sweet Secodammarane Glycosides from Pterocarya paliurus. J. Agric. Food Chem. 1995, 43, 2602–2607. [Google Scholar] [CrossRef]
- Li, C.; Lin, L.-M.; Sui, F.; Wang, Z.-M.; Huo, H.-R.; Dai, L.; Jiang, T.-L. Chemistry and pharmacology of Siraitia grosvenorii: A review. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar] [CrossRef]
- Niu, B.; Ke, C.-Q.; Li, B.-H.; Li, Y.; Yi, Y.; Luo, Y.; Shuai, L.; Yao, S.; Lin, L.-G.; Li, J.; et al. Cucurbitane Glucosides from the Crude Extract of Siraitia grosvenorii with Moderate Effects on PGC-1α Promoter Activity. J. Nat. Prod. 2017, 80, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.S.; Krynitsky, A.J.; Rader, J.I. Sweeteners from plants—with emphasis on Stevia rebaudiana (Bertoni) and Siraitia grosvenorii (Swingle). Anal. Bioanal. Chem. 2013, 405, 4397–4407. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of Siraitia grosvenori (Cucurbitaceae). Chem. Pharm. Bull. 1990, 38, 2030–2032. [Google Scholar] [CrossRef] [Green Version]
- Kasai, R.; Matsumoto, K.; Nie, R.L.; Zhou, J.; Tanaka, O. Glycosides from Chinese medicinal plant, Hemsleya panacis-scandens, and structure-taste relationship to cucurbitane glycosides. Chem. Pharm. Bull. 1988, 36, 234–243. [Google Scholar] [CrossRef]
- Jizba, J.; Dolejš, L.; Herout, V.; Šorm, F. The structure of osladin - the sweet principle of the rhizomes of polypodium vulgare L. Tetrahedron Lett. 1971, 12, 1329–1332. [Google Scholar] [CrossRef]
- Kim, J.; Pezzuto, J.M.; Soejarto, D.D.; Lang, F.A.; Kinghorn, A.D. Polypodoside A, an Intensely Sweet Constituent of the Rhizomes of Polypodium glycyrrhiza. J. Nat. Prod. 1988, 51, 1166–1172. [Google Scholar] [CrossRef]
- Kim, J.; Kinghorn, A.D. Further steroidal and flavonoid constituents of the sweet plant, Polypodium glycyrrhiza. Phytochemistry 1989, 28, 1225–1228. [Google Scholar] [CrossRef]
- Sun, G.; Dai, Q.; Zhang, H.-X.; Li, Z.-J.; Du, Z.-Z. New Sweet-Tasting C21-Pregnane Glycosides from Pericarps of Myriopteron extensum. J. Agric. Food Chem. 2016, 64, 9381–9389. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Briand, L.; A De March, C.; Matsunami, H.; Yamashita, A.; Meyerhof, W.; Weyand, S. Structure–Function Relationships of Olfactory and Taste Receptors. Chem. Senses 2018, 43, 81–87. [Google Scholar] [CrossRef] [PubMed]
















| cpd. | Name | Source | RS | c (Sucrose) | Bitterness |
|---|---|---|---|---|---|
| 16 | monatin | Schlerochiton ilicifolius | 1400 | 5 | – |
| 17 | (+)-phyllodulcin | Hydrangea macrophylla | 400 | 3 | + |
| 18 | (+)-phyllodulcin 8-O-glucoside | Hydrangea macrophylla | |||
| 19 | (+)-diyhdroquercetin 3-acetate | Pluchea dodoneifolia | 80 | 2 | |
| 20 | (+)-diyhdro-6-methoxy-kaempferol 3-acetate | Tetraneuris turneri | 25 | 2 | |
| 21 | (+)-diyhdro-6-methoxy-luetolin 3-acetate | Tetraneuris turneri | 15 | 2 | |
| 22 | (+)-diyhdro-6-methoxy-luteolin | Tetraneuris turneri | 20 | 2 | |
| 23 | glycyphyllin | Smilax leucophylla | ++ | ||
| 24 | phlorizin | Symplocos lancifolia | |||
| 25 | trilobatin | Symplocos microcalyx | |||
| 26 | naringin | Citrus paradisi | +++ | ||
| 27 | neohesperidin | Citrus x aurantium | ++ | ||
| 28 | (+)-haematoxylin | Haematoxylum campechianum | 120 | 3 | |
| 29 | selligueain A | Selliguea feei | 35 | 2 | + |
| 30 | perillaldehyde | Perilla frutescens | |||
| 31 | (+)-hernandulcin | Phyla scaberrima | 1000 | ++ | |
| 32 | (+)-4β-hydroxyhernandulcin | Phyla scaberrima | n.d. | ||
| 33 | mukurozioside IIb | Sapindus rarak | 1 | ||
| 34 | steviolbioside | Stevia rebaudiana | 100 | 10 | + |
| 35 | stevioside | Stevia rebaudiana | 210 | 0.6 | + |
| 36 | rebaudioside E | Stevia rebaudiana | 174 | + | |
| 37 | rebaudioside B | Stevia rebaudiana | 150 | 10 | + |
| 38 | rebaudioside A | Stevia rebaudiana | 242 | + | |
| 39 | rebaudioside D | Stevia rebaudiana | 221 | + | |
| 40 | rebaudioside C | Stevia rebaudiana | 30 | + | |
| 41 | rebaudioside F | Stevia rebaudiana | 200 | + | |
| 42 | dulcoside A | Stevia rebaudiana | 30 | 30 | + |
| 43 | rubusoside | Rubus chingii | 114 | + | |
| 44 | suavioside B | Rubus chingii | |||
| 45 | suavioside G | Rubus chingii | |||
| 46 | suavioside H | Rubus chingii | |||
| 47 | suavioside I | Rubus chingii | |||
| 48 | suavioside J | Rubus chingii | |||
| 49 | bayunoside | Phlomoides betonicoides | 500 | ||
| 50 | gaudichaudioside A | Baccharis gaudichaudiana | 55 | 2 | – |
| 51 | gaudichaudioside B | Baccharis gaudichaudiana | ++ | ||
| 52 | gaudichaudioside E | Baccharis gaudichaudiana | ++ | ||
| 53 | 4β,10α-dimethyl-1,2,3,4,5,10-hexahydrofluorene-4α,6-dicarboxylic acid | Pinus sp. | 1600 | +++ | |
| 54 | glycyrrhizic acid | Glycyrrhiza glabra | 93–170 | ++ | |
| 55 | albiziasaponin A | Albizia myriophylla | 5 | ||
| 56 | albiziasaponin B | Albizia myriophylla | 600 | ||
| 57 | periandrin I | Periandra mediterranea | 93–170 | + | |
| 58 | periandrin III | Periandra mediterranea | 93–170 | + | |
| 59 | periandrin V | Periandra mediterranea | 200 | + | |
| 60 | periandrin II | Periandra mediterranea | 93–170 | + | |
| 61 | periandrin IV | Periandra mediterranea | 93–170 | ||
| 62 | abrusoside A | Abrus precatorius | 30 | – | |
| 63 | abrusoside B | Abrus precatorius | 100 | – | |
| 64 | abrusoside C | Abrus precatorius | 50 | – | |
| 65 | abrusoside D | Abrus precatorius | 75 | – | |
| 66 | abrusoside E | Abrus precatorius | |||
| 67 | cyclocarioside A | Cyclocarya paliurus | 200 | ||
| 68 | cyclocarioside I | Cyclocarya paliurus | 250 | ||
| 69 | pterocaryoside A | Cyclocarya paliurus | 50 | 2 | + |
| 70 | pterocaryoside B | Cyclocarya paliurus | 100 | 2 | + |
| 71 | mogroside III | Siraitia grosvenorii | – | ||
| 72 | mogroside IIIA1 | Siraitia grosvenorii | – | ||
| 73 | mogroside IIIA2 | Siraitia grosvenorii | – | ||
| 74 | mogroside IIIE | Siraitia grosvenorii | – | ||
| 75 | mogroside IVA | Siraitia grosvenorii | – | ||
| 76 | mogroside IVE | Siraitia grosvenorii | 392 | 1 | – |
| 77 | siamenoside I | Siraitia grosvenorii | 563 | 1 | – |
| 78 | grosmomoside | Siraitia grosvenorii | – | ||
| 79 | mogroside V | Siraitia grosvenorii | 425 | 1 | – |
| 80 | isomogroside V | Siraitia grosvenorii | – | ||
| 81 | neomogroside | Siraitia grosvenorii | – | ||
| 82 | mogroside VI | Siraitia grosvenorii | – | ||
| 83 | mogroside VIA | Siraitia grosvenorii | – | ||
| 84 | mogroside VIB | Siraitia grosvenorii | – | ||
| 85 | 7-oxomogroside IIIE | Siraitia grosvenorii | |||
| 86 | 7-oxomogroside IV | Siraitia grosvenorii | |||
| 87 | 7-oxomogroside V | Siraitia grosvenorii | |||
| 88 | osladin | Polypodium vulgare | 500 | + | |
| 89 | polypodoside A | Polypodium glycyrrhiza | 600 | 6 | + |
| 90 | polypodoside B | Polypodium glycyrrhiza | |||
| 91 | extensumside C | Myriopteron extensum | 400 | 1 | |
| 92 | extensumside E | Myriopteron extensum | 200 | 1 | |
| 93 | extensumside F | Myriopteron extensum | 200 | 1 | |
| 94 | extensumside H | Myriopteron extensum | 200 | 1 | |
| 95 | extensumside D | Myriopteron extensum | 300 | 1 | |
| 96 | extensumside G | Myriopteron extensum | 100 | 1 | |
| 97 | extensumside I | Myriopteron extensum | 150 | 1 | |
| 98 | extensumside J | Myriopteron extensum | 100 | 1 | |
| 99 | extensumside K | Myriopteron extensum | 50 | 1 | |
| 100 | extensumside L | Myriopteron extensum | 50 | 1 |
| cpd. | Name | Derived From | RS | c (Sucrose) | Bitterness |
|---|---|---|---|---|---|
| 5a | sucralose | sucrose | |||
| 17a | 2-(3-hydroxy-4-methoxypheyl)-1,3-benzodioxan | phyllodulcin | 3000 | ||
| 17b | 2-(3-hydroxy-4-methoxypheyl)-1,4-benzodioxan | phyllodulcin | 450 | ||
| 17c | (+)-2-(3-hydroxy-4-methoxypheyl)-1,3-benzoxathian | phyllodulcin | 18000 | ||
| 17d | (+)-2-(3-hydroxy-4-methoxypheyl)-1,3-benzodithian | phyllodulcin | 20000 | ||
| 19a | 4′-methyldihydro-quercetin 3-acetate | (+)-diyhdroquercetin 3-acetate | 400 | 2 | – |
| 26a | naringin dihydrochalcone | naringin | 300 | 5 | |
| 27a | neohesperidin dihydrochalcone | neohesperidin | 1000 | 5 | + |
| 27b | 3′carboxy-hesperetin | neohesperidin | 3400 | 6 | + |
| 28a | (6aS,11aS)-8-methoxy-11a-methyl-6,11-dihydroindeno[1,2-c]chromene-6a,9-diol | (+)-haematoxylin | 50 | 3 | |
| 30a | perillartine | perillaldehyde | 370 | ++ | |
| 30b | 4-(methoxymethyl)-1,4-cyclohexadiene-1-carboxaldehyde syn-oxime | perillaldehyde | 450 | ||
| 35a | steviolbioside sulfopropyl ester sodium salt | stevioside | 160 | + | |
| 35b | steviolbioside sulfoprop-2-yl ester sodium salt | stevioside | 120 | – | |
| 38a | rebaudioside B sulfopropyl ester sodium salt | rebaudioside A | 170 | – | |
| 43a | 13-O-β-maltotriosyl-19-O-β-d-glucosylsteviol | rubusoside | 298 | – | |
| 66a | abrusoside E 6′’-O-methyl ester | abrusoside E | 150 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ҫiçek, S.S. Structure-Dependent Activity of Plant-Derived Sweeteners. Molecules 2020, 25, 1946. https://doi.org/10.3390/molecules25081946
Ҫiçek SS. Structure-Dependent Activity of Plant-Derived Sweeteners. Molecules. 2020; 25(8):1946. https://doi.org/10.3390/molecules25081946
Chicago/Turabian StyleҪiçek, Serhat Sezai. 2020. "Structure-Dependent Activity of Plant-Derived Sweeteners" Molecules 25, no. 8: 1946. https://doi.org/10.3390/molecules25081946