An Oxalate-Bridged Copper(II) Complex Combining Monodentate Benzoate, 2,2′-bipyridine and Aqua Ligands: Synthesis, Crystal Structure and Investigation of Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure
2.2. Thermal Analysis
2.3. Vibrational Spectroscopy
2.4. Electron Paramagnetic Resonance Spectroscopy (EPR)
2.5. UV/Vis Spectroscopy
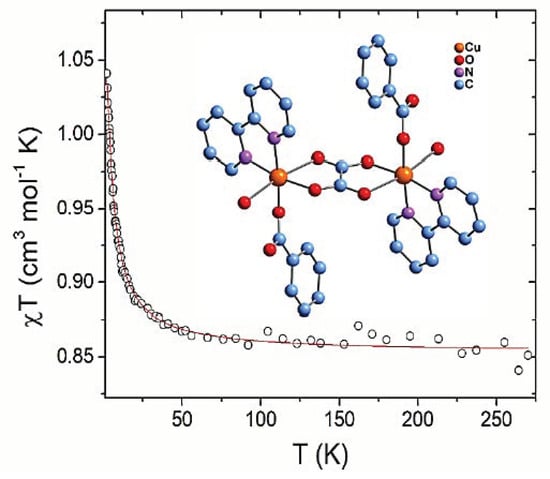
2.6. Magnetic Properties and ab Initio Calculations
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of Compound 1
3.3. X-ray Crystallographic Data Collection and Refinement
3.4. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, K.-B.; Chen, Z.-F.; Liu, Y.-C.; Xie, X.-L.; Liang, H. Dihydroisoquinoline copper(II) complexes: Crystal structures, cytotoxicity, and action mechanism. Rsc. Adv. 2015, 5, 81313–81323. [Google Scholar] [CrossRef]
- Usman, M.; Arjmand, F.; Khan, R.A.; Alsalme, A.; Ahmad, M.; Bishwas, M.S.; Tabassum, S. Tetranuclear cubane Cu4O4 complexes as prospective anticancer agents: Design, synthesis, structural elucidation, magnetism, computational and cytotoxicity studies. Inorg. Chim. Acta 2018, 473, 121–132. [Google Scholar] [CrossRef]
- Sanyal, R.; Kundu, P.; Rychagova, E.; Zhigulin, G.; Ketkov, S.; Ghosh, B.; Chattopadhyay, S.K.; Zangrando, E.; Das, D. Catecholase activity of Mannich-based dinuclear CuII complexes with theoretical modeling: New insight into the solvent role in the catalytic cycle. New J. Chem. 2016, 40, 6623–6635. [Google Scholar] [CrossRef]
- Parween, A.; Naskar, S.; Mota, A.J.; Espinosa Ferao, A.; Chattopadhyay, S.K.; Rivière, E.; Lewis, W.; Naskar, S. C i -Symmetry, [2 × 2] grid, square copper complex with the N4,N5-bis(4-fluorophenyl)-1H-imidazole-4,5-dicarboxamide ligand: Structure, catecholase activity, magnetic properties and DFT calculations. New J. Chem. 2017, 41, 11750–11758. [Google Scholar] [CrossRef] [Green Version]
- Castro, I.; Calatayud, M.L.; Yuste, C.; Castellano, M.; Ruiz-García, R.; Cano, J.; Faus, J.; Verdaguer, M.; Lloret, F. Dinuclear copper(II) complexes as testing ground for molecular magnetism theory. Polyhedron 2019, 169, 66–77. [Google Scholar] [CrossRef]
- Dutta, D.; Nath, H.; Frontera, A.; Bhattacharyya, M.K. A novel oxalato bridged supramolecular ternary complex of Cu(II) involving energetically significant π-hole interaction: Experimental and theoretical studies. Inorg. Chim. Acta 2019, 487, 354–361. [Google Scholar] [CrossRef]
- Calatayud, M.A.L.; Castro, I.; Sletten, J.; Lloret, F.; Julve, M. Syntheses, crystal structures and magnetic properties of chromato-, sulfato-, and oxalato-bridged dinuclear copper(II) complexes. Inorg. Chim. Acta 2000, 300–302, 846–854. [Google Scholar] [CrossRef]
- Cangussu, D.; Stumpf, H.O.; Adams, H.; Thomas, J.A.; Lloret, F.; Julve, M. Oxalate, squarate and croconate complexes with bis(2-pyrimidylcarbonyl)amidatecopper(II): Synthesis, crystal structures and magnetic properties. Inorg. Chim. Acta 2005, 358, 2292–2302. [Google Scholar] [CrossRef]
- Zheng, A.-L.; Ju, Z.-F.; Li, W.; Zhang, J. Ferromagnetic dinuclear copper(II) complex based on bridging oxalate and bipyridinium ligands. Inorg. Chem. Commun. 2006, 9, 489–492. [Google Scholar] [CrossRef]
- Castillo, O.; Luque, A.; Román, P.; Lloret, F.; Julve, M. Syntheses, Crystal Structures, and Magnetic Properties of One-Dimensional Oxalato-Bridged Co(II), Ni(II), and Cu(II) Complexes with n-Aminopyridine (n = 2−4) as Terminal Ligand. Inorg. Chem. 2001, 40, 5526–5535. [Google Scholar] [CrossRef]
- Julve, M.; Gleizes, A.; Chamoreau, L.M.; Ruiz, E.; Verdaguer, M. Antiferromagnetic Interactions in Copper(II) µ-Oxalato Dinuclear Complexes: The Role of the Counterion. Eur. J. Inorg. Chem. 2018, 2018, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.-Y.; Chi, Y.-H.; Liang, Y.; Cottrill, E.; Pan, N.; Shi, J.-M. Green and mild oxidation: From acetate anion to oxalate anion. J. Coord. Chem. 2018, 71, 3947–3954. [Google Scholar] [CrossRef]
- Carranza, J.; Brennan, C.; Sletten, J.; Vangdal, B.; Rillema, P.; Lloret, F.; Julve, M. Syntheses, crystal structures and magnetic properties of new oxalato-, croconato- and squarato-containing copper(II) complexes. New J. Chem. 2003, 27, 1775–1783. [Google Scholar] [CrossRef]
- Melnic, E.; Kravtsov, V.C.; Krämer, K.; van Leusen, J.; Decurtins, S.; Liu, S.-X.; Kögerler, P.; Baca, S.G. Versatility of copper(II) coordination compounds with 2,3-bis(2-pyridyl)pyrazine mediated by temperature, solvents and anions choice. Solid State Sci. 2018, 82, 1–12. [Google Scholar] [CrossRef]
- Golchoubian, H.; Samimi, R. Solvato- and thermochromism study in oxalato-bridged dinuclear copper(II) complexes of bidentate diamine ligands. Polyhedron 2017, 128, 68–75. [Google Scholar] [CrossRef]
- Royappa, A.T.; Royappa, A.D.; Moral, R.F.; Rheingold, A.L.; Papoular, R.J.; Blum, D.M.; Duong, T.Q.; Stepherson, J.R.; Vu, O.D.; Chen, B.; et al. Copper(I) oxalate complexes: Synthesis, structures and surprises. Polyhedron 2016, 119, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Świtlicka-Olszewska, A.; Machura, B.; Mroziński, J.; Kalińska, B.; Kruszynski, R.; Penkala, M. Effect of N-donor ancillary ligands on structural and magnetic properties of oxalate copper(ii) complexes. New J. Chem. 2014, 38, 1611–1626. [Google Scholar] [CrossRef] [Green Version]
- Miyazato, Y.; Asato, E.; Ohba, M.; Wada, T. Synthesis and Characterization of a Di-µ-oxalato Tetracopper(II) Complex with Tetranucleating Macrocyclic Ligand. B. Chem. Soc. Jpn 2016, 89, 430–436. [Google Scholar] [CrossRef]
- Yuste, C.; Cañadillas-Delgado, L.; Labrador, A.; Delgado, F.S.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Low-Dimensional Copper(II) Complexes with the Trinucleating Ligand 2,4,6-Tris(di-2-pyridylamine)-1,3,5-triazine: Synthesis, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2009, 48, 6630–6640. [Google Scholar] [CrossRef]
- Vicente, R.; Escuer, A.; Solans, X.; Font-Bardía, M. Synthesis, magnetic behaviour and structural characterization of the alternating hexanuclear copper(II) compound [Cu6(tmen)6(µ-N3)2(µ-C2O4)3(H2O)2][ClO4]4·2H2O (tmen = Me2NCH2CH2NMe2). J. Chem. Soc. Dalton Trans. 1996, 1996, 1835–1838. [Google Scholar] [CrossRef]
- Nakahata, D.H.; de Paiva, R.E.F.; Lustri, W.R.; Ribeiro, C.M.; Pavan, F.R.; da Silva, G.G.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Corbi, P.P. Sulfonamide-containing copper(II) metallonucleases: Correlations with in vitro antimycobacterial and antiproliferative activities. J. Inorg. Biochem. 2018, 187, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxim, C.; Ferlay, S.; Train, C. Binuclear heterometallic M(III)–Mn(II) (M = Fe, Cr) oxalate-bridged complexes associated with a bisamidinium dication: A structural and magnetic study. New J. Chem. 2011, 35, 1254–1259. [Google Scholar] [CrossRef]
- Glerup, J.; Goodson, P.A.; Hodgson, D.J.; Michelsen, K. Magnetic Exchange through Oxalate Bridges: Synthesis and Characterization of (mu-Oxalato)dimetal(II) Complexes of Manganese, Iron, Cobalt, Nickel, Copper, and Zinc. Inorg. Chem. 1995, 34, 6255–6264. [Google Scholar] [CrossRef]
- Baruah, B.; Golub, V.O.; O’Connor, C.J.; Chakravorty, A. Synthesis of Oxalato-Bridged (Oxo)vanadium(IV) Dimers Using L-Ascorbic Acid as Oxalate Precursor: Structure and Magnetism of Two Systems. Eur. J. Inorg. Chem. 2003, 2003, 2299–2303. [Google Scholar] [CrossRef]
- Bratsos, I.; Serli, B.; Zangrando, E.; Katsaros, N.; Alessio, E. Replacement of Chlorides with Dicarboxylate Ligands in Anticancer Active Ru(II)-DMSO Compounds: A New Strategy That Might Lead to Improved Activity. Inorg. Chem. 2007, 46, 975–992. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V.; Bencini, A.; Totti, F.; Ciofini, I. On the Calculation and Modeling of Magnetic Exchange Interactions in Weakly Bonded Systems: The Case of the Ferromagnetic Copper(II) μ2-Azido Bridged Complexes. Inorg. Chem. 1999, 38, 1996–2004. [Google Scholar] [CrossRef]
- Calzado, C.J.; Cabrero, J.; Malrieu, J.P.; Caballol, R. Analysis of the magnetic coupling in binuclear complexes. I. Physics of the coupling. J. Chem. Phys. 2002, 116, 2728–2747. [Google Scholar] [CrossRef]
- Calzado, C.J.; Cabrero, J.; Malrieu, J.P.; Caballol, R. Analysis of the magnetic coupling in binuclear complexes. II. Derivation of valence effective Hamiltonians from ab initio CI and DFT calculations. J. Chem. Phys. 2002, 116, 3985–4000. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Thompson, L.K.; Miller, D.O. Dicopper(II) Complexes Bridged by Single N−N Bonds. Magnetic Exchange Dependence on the Rotation Angle between the Magnetic Planes. Inorg. Chem. 1997, 36, 3985–3995. [Google Scholar] [CrossRef]
- Thompson, L.K.; Tandon, S.S.; Lloret, F.; Cano, J.; Julve, M. An Azide-Bridged Copper(II) Ferromagnetic Chain Compound Exhibiting Metamagnetic Behavior. Inorg. Chem. 1997, 36, 3301–3306. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Jurić, M.; Pajić, D.; Žilić, D.; Rakvin, B.; Milić, D.; Planinić, P. Synthesis, crystal structures and magnetic properties of the oxalate-bridged single CuIICuII and cocrystallized CuIIZnII systems. Three species (CuCu, CuZn, ZnZn) in the crystalline lattice. Polyhedron 2015, 98, 26–34. [Google Scholar]
- Goswami, S.; Singha, S.; Saha, R.; Singha Roy, A.; Islam, M.; Kumar, S. A bi-nuclear Cu(II)-complex for selective epoxidation of alkenes: Crystal structure, thermal, photoluminescence and cyclic voltammetry. Inorg. Chim. Acta 2019, 486, 352–360. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pereira, C.L.M.; Pinheiro, C.B.; Lloret, F.; Julve, M. Oxotris(oxalate)niobate(V): An oxalate delivery agent in the design of building blocks. J. Coord. Chem. 2018, 71, 707–724. [Google Scholar] [CrossRef]
- Samimi, R.; Golchoubian, H. Dinuclear copper(II) complexes with ethylenediamine derivative and bridging oxalato ligands: Solvatochromism and density functional theory studies. Transit. Met. Chem. 2017, 42, 643–653. [Google Scholar] [CrossRef]
- Calatayud, M.L.; Orts-Arroyo, M.; Julve, M.; Lloret, F.; Marino, N.; De Munno, G.; Ruiz-García, R.; Castro, I. Magneto-structural correlations in asymmetric oxalato-bridged dicopper(II) complexes with polymethyl-substituted pyrazole ligands. J. Coord. Chem. 2018, 71, 657–674. [Google Scholar] [CrossRef]
- Bahemmat, S.; Neumüller, B.; Ghassemzadeh, M. One-Pot Synthesis of an Oxalato-Bridged CuII Coordination Polymer Containing an in Situ Produced Pyrazole Moiety: A Precursor for the Preparation of CuO Nano structures. Eur. J. Inorg. Chem. 2015, 2015, 4116–4124. [Google Scholar] [CrossRef]
- Liu, C.; Abboud, K.A. Crystal structures of [mu]-oxalato-bis[azido(histamine)copper(II)] and [mu]-oxalato-bis[(dicyanamido)(histamine)copper(II)]. Acta Cryst. 2015, E71, 1379–1383. [Google Scholar]
- Min, K.S.; Suh, M.P. Self-Assembly, Structures, and Magnetic Properties of Ladder-Like Copper(II) Coordination Polymers. J. Solid State Chem. 2000, 152, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Hökelek, T.; Ünaleroğglu, C.; Mert, Y. Crystal Structure of [Bis(N,N,N’,N’-tetramethylethylenediamine)-O,O’-μ-O,O’-oxalato]dihydroxy Dicopper(II). Anal. Sci. 2000, 16, 1235–1236. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Ogawa, T. Spectral and structural studies of a new oxalato-bridged dinuclear copper(II) complex having two 3-(thiophen-2-yl)-1,10-phenanthroline ligands in a trans configuration. Inorg. Chim. Acta 2009, 362, 3877–3880. [Google Scholar] [CrossRef]
- Thuéry, P. Increasing Complexity in the Uranyl Ion–Kemp’s Triacid System: From One- and Two-Dimensional Polymers to Uranyl–Copper(II) Dodeca- and Hexadecanuclear Species. Cryst. Growth Des. 2014, 14, 2665–2676. [Google Scholar] [CrossRef]
- Du, M.; Guo, Y.-M.; Chen, S.-T.; Bu, X.-H.; Ribas, J. Crystal structures, spectra and magnetic properties of di-2-pyridylamine (dpa) CuII complexes [Cu(dpa)2(N3)2]·(H2O)2 and [Cu2(μ-ox)(dpa)2(CH3CN)2](ClO4)2. Inorg. Chim. Acta 2003, 346, 207–214. [Google Scholar] [CrossRef]
- Youngme, S.; van Albada, G.A.; Chaichit, N.; Gunnasoot, P.; Kongsaeree, P.; Mutikainen, I.; Roubeau, O.; Reedijk, J.; Turpeinen, U. Synthesis, spectroscopic characterization, X-ray crystal structure and magnetic properties of oxalato-bridged copper(II) dinuclear complexes with di-2-pyridylamine. Inorg. Chim. Acta 2003, 353, 119–128. [Google Scholar] [CrossRef]
- Castillo, O.; Muga, I.; Luque, A.; Gutiérrez-Zorrilla, J.M.; Sertucha, J.; Vitoria, P.; Román, P. Synthesis, chemical characterization, X-ray crystal structure and magnetic properties of oxalato-bridged copper(II) binuclear complexes with 2,2′-bipyridine and diethylenetriamine as peripheral ligands. Polyhedron 1999, 18, 1235–1245. [Google Scholar] [CrossRef]
- Gusev, A.N.; Nemec, I.; Herchel, R.; Bayjyyev, E.; Nyshchimenko, G.A.; Alexandrov, G.G.; Eremenko, I.L.; Trávníček, Z.; Hasegawa, M.; Linert, W. Versatile coordination modes of bis [5-(2-pyridine-2-yl)-1,2,4-triazole-3-yl]alkanes in Cu(ii) complexes. Dalton Trans. 2014, 43, 7153–7165. [Google Scholar] [CrossRef] [Green Version]
- Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W. Reduction of carbon dioxide to oxalate by a binuclear copper complex. Nat. Commun. 2014, 5, 5883. [Google Scholar] [CrossRef] [Green Version]
- Boyko, A.N.; Haukka, M.; Golenya, I.A.; Pavlova, S.V.; Usenko, N.I. [mu]-Oxalato-bis[(2,2’-bipyridyl)copper(II)] bis(perchlorate) dimethylformamide disolvate monohydrate. Acta Cryst. 2010, E66, m1101–m1102. [Google Scholar]
- Castro, I.; Faus, J.; Julve, M.; Mollar, M.; Monge, A.; Gutierrez-Puebla, E. Formation in solution, synthesis and crystal structure of μ-oxalatobis[bis(2-pyridylcarbonyl)amido] dicopper(II). Inorg. Chim. Acta 1989, 161, 97–104. [Google Scholar] [CrossRef]
- Li, K.-k.; Zhang, C.; Xu, W. [mu]-Oxalato-bis[bis(2,2’-bipyridine)manganese(II)] bis(perchlorate) 2,2’-bipyridine solvate. Acta Cryst. 2011, E67, m1443–m1444. [Google Scholar]
- Kaizaki, S.; Nakahanada, M.; Fuyuhiro, A.; Ikedo-Urade, M.; Abe, Y. Synthesis, characterization and redox reactivity of l-tartrato bridged dinuclear manganese complex with 2,2′-bipyridine. Inorg. Chim. Acta 2009, 362, 5117–5121. [Google Scholar] [CrossRef]
- Duan, W.; Jiao, S.; Liu, X.; Chen, J.; Cao, X.; Chen, Y.; Xu, W.; Cui, X.; Xu, J.; Pang, G. Two new supramolecular hybrids based on bi-capped Keggin {PMo12V2O42} clusters and transition metal mixed-organic-ligand complexes. Chem. Res. Chin. Univ. 2015, 31, 179–186. [Google Scholar] [CrossRef]
- Shi, C.; Fan, L.; Wei, P.; Li, B.; Zhang, X. (μ-Oxalato-κ4O1,O2:O1’,O2’)bis[bis(2,2’-bipyridine-κ2N,N’)cobalt(II)] μ6-oxido-dodeca-μ2-oxido-hexaoxido-hexatungstate(VI). Acta Cryst. 2010, E66, m822–m823. [Google Scholar] [CrossRef] [PubMed]
- Androš Dubraja, L.; Jurić, M.; Torić, F.; Pajić, D. The influence of metal centres on the exchange interaction in heterometallic complexes with oxalate-bridged cations. Dalton Trans. 2017, 46, 11748–11756. [Google Scholar] [CrossRef] [PubMed]
- Youngme, S.; Gunnasoot, P.; Chaichit, N.; Pakawatchai, C. Dinuclear copper(II) complexes with ferromagnetic and antiferromagnetic interactions mediated by a bridging oxalato group: Structures and magnetic properties of [Cu2L4(μ-C2O4)](PF6)2(H2O)2 and [Cu2L2(μ-C2O4)(NO3)2((CH3)2NCOH)2] (L = di-2-pyridylamine). Transit. Met. Chem. 2004, 29, 840–846. [Google Scholar] [CrossRef]
- Qin, C. μ-Oxalato-κ2O1,O2:κ2O1’,O2’-bis[(3,5-dicarboxybenzoato-κ2O1,O1’)(1,10-phenanthroline-κ2N,N’)copper(II)]. Acta Cryst. 2007, E63, m1006–m1007. [Google Scholar] [CrossRef]
- de Faria, D.M.; Yoshida, M.I.; Pinheiro, C.B.; Guedes, K.J.; Krambrock, K.; Diniz, R.; de Oliveira, L.F.C.; Machado, F.C. Preparation, crystal structures and spectroscopic characterization of oxalate copper(II) complexes containing the nitrogen ligands 4,4′-dimethyl-2,2′-bipyridine and di(2-pyridyl)sulfide. Polyhedron 2007, 26, 4525–4532. [Google Scholar] [CrossRef]
- Androš, L.; Jurić, M.; Planinić, P.; Žilić, D.; Rakvin, B.; Molčanov, K. New mononuclear oxalate complexes of copper(II) with 2D and 3D architectures: Synthesis, crystal structures and spectroscopic characterization. Polyhedron 2010, 29, 1291–1298. [Google Scholar] [CrossRef]
- Sarma, R.; Boudalis, A.K.; Baruah, J.B. Aromatic N-oxide bridged copper(II) coordination polymers: Synthesis, characterization and magnetic properties. Inorg. Chim. Acta 2010, 363, 2279–2286. [Google Scholar] [CrossRef]
- Stepanian, S.G.; Reva, I.D.; Radchenko, E.D.; Sheina, G.G. Infrared spectra of benzoic acid monomers and dimers in argon matrix. Vib. Spectrosc. 1996, 11, 123–133. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds—Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Edwards, H.G.M.; Farwell, D.W.; Rose, S.J.; Smith, D.N. Vibrational spectra of copper (II) oxalate dihydrate, CuC2O4·2H20, and dipotassium bis-oxalato copper (II) tetrahydrate, K2Cu(C2O4)2·4H2O. J. Mol. Struc. 1991, 249, 233–243. [Google Scholar] [CrossRef]
- Halaška, J.; Pevec, A.; Strauch, P.; Kozlevčar, B.; Koman, M.; Moncol, J. Supramolecular hydrogen-bonding networks constructed from copper(II) chlorobenzoates with nicotinamide: Structure and EPR. Polyhedron 2013, 61, 20–26. [Google Scholar] [CrossRef]
- Godlewska, S.; Jezierska, J.; Baranowska, K.; Augustin, E.; Dołęga, A. Copper(II) complexes with substituted imidazole and chlorido ligands: X-ray, UV–Vis, magnetic and EPR studies and chemotherapeutic potential. Polyhedron 2013, 65, 288–297. [Google Scholar] [CrossRef]
- Vicente, R.; Escuer, A.; Ferretjans, J.; Stoeckli-Evans, H.; Solans, X.; Font-Bardía, M. Structurally alternating copper(II) chains from oxalate and azide bridging ligands: Syntheses and crystal structure of [Cu2(µ-ox)(deen)2(H2O)2(ClO4)2] and [{Cu2(µ-N3)(µ-ox)(deen)2}n][ClO4] n (deen = Et2NCH2CH2NH2). J. Chem. Soc. Dalton Trans. 1997, 1997, 167–172. [Google Scholar] [CrossRef]
- Recio, A.; Server-Carrió, J.; Escrivà, E.; Acerete, R.; García-Lozano, J.; Sancho, A.; Soto, L. Novel Cu(II)-Based Frameworks Built from BIMAM and Oxalate: Syntheses, Structures, and Magnetic Characterizations (BIMAM = Bis(imidazol-yl) methylaminomethane). Cryst. Growth Des. 2008, 8, 4075–4082. [Google Scholar] [CrossRef]
- Halcrow, M.A. Interpreting and controlling the structures of six-coordinate copper(II) centres—When is a compression really a compression? Dalton Trans. 2003, 4375–4384. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Hamamci, S.; Andac, O.; Thöne, C.; Harrison, W.T.A. Mono- and binuclear copper(II) complexes of saccharin with 2-pyridinepropanol synthesis, spectral, thermal and structural characterization. Transit. Met. Chem. 2003, 28, 676–681. [Google Scholar] [CrossRef]
- zuah, R.T.; LR, K.; Qiu, Y.; Tregenna-Piggott, P.L.W.; Brown, C.M.; Copley, J.R.D.; Dimeo, R.M. DAVE: A comprehensive software suite for the reduction, visualization, and analysis of low energy neutron spectroscopic data. J. Res. Nat. Inst. Stan. 2009, 114, 341–358. [Google Scholar]
- Bencini, A.; Gatteschi, D. Electron Paramagnetic Resonance of Exchange Coupled Systems; Springer-Verlag: Berlin, Germany, 1990. [Google Scholar]
- Pal, P.; Konar, S.; El Fallah, M.S.; Das, K.; Bauzá, A.; Frontera, A.; Mukhopadhyay, S. Synthesis, crystal structures, magnetic properties and DFT calculations of nitrate and oxalate complexes with 3,5 dimethyl-1-(2′-pyridyl)-pyrazole-Cu(ii). Rsc Adv. 2015, 5, 45082–45091. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. App. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wires Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Bencini, A.; Totti, F.; Daul, C.A.; Doclo, K.; Fantucci, P.; Barone, V. Density Functional Calculations of Magnetic Exchange Interactions in Polynuclear Transition Metal Complexes. Inorg. Chem. 1997, 36, 5022–5030. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Program APEX3; Bruker AXS Inc.: Madison, WI, USA, 2015; Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/apex3.html (accessed on 16 April 2020).
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. App. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Bencini, A.; Totti, F. A Few Comments on the Application of Density Functional Theory to the Calculation of the Magnetic Structure of Oligo-Nuclear Transition Metal Clusters. J. Chem. Theory Comput. 2009, 5, 144–154. [Google Scholar] [CrossRef]
- Neese, F. Efficient and accurate approximations to the molecular spin-orbit coupling operator and their use in molecular g-tensor calculations. J. Chem. Phys. 2005, 122, 034107. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound 1 is available from the authors. |








| Bond Length (Å) | |||
| Cu(1)–O(1) | 2.426(3) | Cu(1)–N(2) | 2.006(2) |
| Cu(1)–O(5) | 1.9732(19) | O(6)–C(11) | 1.263(3) |
| Cu(1)–O(8) | 2.378(2) | O(7)–C(11) | 1.230(3) |
| Cu(1)–O(6) | 1.9425(19) | C(18)–O(5) | 1.260(3) |
| Cu(1)–N(1) | 2.008(2) | C(18) i–O(8) | 1.230(3) |
| Bond Angle (°) | |||
| O(5)–Cu(1)–O(8) | 76.62(7) | O(5)–Cu(1)–N(2) | 93.67(8) |
| O(6)–Cu(1)–O(1) | 89.46(9) | N(1)–Cu(1)–O(8) | 95.24(7) |
| O(1)–Cu(1)–O(5) | 94.26(8) | N(2)–Cu(1)–O(8) | 94.08(8) |
| O(1)–Cu(1)–O(8) | 170.00(7) | N(1)–Cu(1)–N(2) | 80.56(9) |
| O(6)–Cu(1)–O(5) | 92.77(9) | N(1)–Cu(1)–O(1) | 94.26(8) |
| O(6)–Cu(1)–O(8) | 86.93(9) | N(2)–Cu(1)–O(1) | 90.55(9) |
| O(6)–Cu(1)–N(1) | 93.00(9) | H(1B)–O(1)–H(1A) | 104(3) |
| O(6)–Cu(1)–N(2) | 173.55(9) | O(8) i–C(18)–O(5) | 124.4(2) |
| O(5)–Cu(1)–N(1) | 169.75(9) | O(7)–C(11)–O(6) | 125.7(3) |
| Sample | g-Matrix | A-Matrix/MHz | D-Matrix/MHz | |||||
|---|---|---|---|---|---|---|---|---|
| gx | gy | gz | Ax | Ay | Az | D | E | |
| Solid | 2.079 | 2.079 | 2.278 | <100 | <100 | >360 | 350 | 65.5 |
| Frozen solution | 2.053 | 2.078 | 2.262 | <100 | <100 | 555.1 | - | - |
| Compound (a) | dCu⋅⋅⋅Cu (Å) (b) | θ (°) (c) | α (°) (d) | J (cm−1) (e) | Reference |
|---|---|---|---|---|---|
| [{Cu(bipy)(bzt)(OH2)}2(μ-ox)] | 5.60 | 85.9 | 107.7 | +2.3 | Complex 1 |
| [{Cu(dpyam)2}2(μ-ox)](BF4)2·3H2O | 5.74 | 86.1 | - | +3.38 | [44] |
| [{Cu(dpyam)2}2(μ-ox)](ClO4)2·3H2O | 5.75 | 77.0 | - | +2.42 | [44] |
| [{Cu(prbipy)}2(μ-ox)]·4H2O | 5.46 | 78.7 | 107.4 | +3.22 | [9] |
| [{Cu(bpca)(H2O)}2(μ-ox)]·2H2O | 5.63 | 80.7 | 106.8 | +1.1 | [7] |
| [{Cu(bpca)}2(μ-ox)] | 5.44 | 86.3 | 107.4 | +1.0 | [7,49] |
| [{Cu(bpcam)(H2O)}2(μ-ox)] | 5.68 | 81.6 | 106.6 | +0.75 | [8] |
| [{Cu(dien)}2(μ-ox)](NO3)2 | 5.14 | 4.6 | 110.2 | −6.5 | [45] |
| [{Cu(3-ampy)}2(μ-ox)]n | 5.46 | 4.8 | 111.0 | −1.3 | [10] |
| [{Cu(4-ampy)}2(μ-ox)]n | 5.66 | 2.4 | 109.7 | −1.1 | [10] |
| Formula | C36H30Cu2N4O10 |
|---|---|
| Formula weight | 805.72 g mol−1 |
| Temperature (K) | 302(2) |
| Wavelength (Å) | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P21/n (equiv. to no 14) |
| Z/calculated density | 2/1.593 Mg m−3 |
| a (Å) | 7.1994(3) |
| b (Å) | 10.0894(4) |
| c (Å) | 23.1941(10) |
| β (°) | 94.385(2) |
| Unit cell volume (Å3) | 1679.83(12) |
| Absorption coefficient μ (mm−1) | 1.333 |
| F(000) | 824 |
| Crystal size (mm)/colour | 0.175 × 0.087 × 0.067/bluish-green |
| θ range (°) | 2.9–25.0 |
| h, k, l ranges | ±8, ±11, ±27 |
| Completeness to θ = 25° | 99.9% |
| Total reflections/unique reflections/Rint | 51619/2953/0.069 |
| No of parameters/restraints | 243/2 |
| Goodness-of-fit on F2 (GOF) | 1.037 |
| R1, wR2 (all data) b | 0.052, 0.085 |
| R1, wR2 [I > 2σ(I)] a,b | 0.033, 0.078 |
| Δρmaximum/Δρminimum(eÅ−3) | 0.29/−0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, F.S.; Briganti, M.; Cassaro, R.A.A.; Totti, F.; Ribeiro, R.R.; Hughes, D.L.; Nunes, G.G.; Reis, D.M. An Oxalate-Bridged Copper(II) Complex Combining Monodentate Benzoate, 2,2′-bipyridine and Aqua Ligands: Synthesis, Crystal Structure and Investigation of Magnetic Properties. Molecules 2020, 25, 1898. https://doi.org/10.3390/molecules25081898
Santana FS, Briganti M, Cassaro RAA, Totti F, Ribeiro RR, Hughes DL, Nunes GG, Reis DM. An Oxalate-Bridged Copper(II) Complex Combining Monodentate Benzoate, 2,2′-bipyridine and Aqua Ligands: Synthesis, Crystal Structure and Investigation of Magnetic Properties. Molecules. 2020; 25(8):1898. https://doi.org/10.3390/molecules25081898
Chicago/Turabian StyleSantana, Francielli Sousa, Matteo Briganti, Rafael A. Allão Cassaro, Federico Totti, Ronny Rocha Ribeiro, David L. Hughes, Giovana Gioppo Nunes, and Dayane Mey Reis. 2020. "An Oxalate-Bridged Copper(II) Complex Combining Monodentate Benzoate, 2,2′-bipyridine and Aqua Ligands: Synthesis, Crystal Structure and Investigation of Magnetic Properties" Molecules 25, no. 8: 1898. https://doi.org/10.3390/molecules25081898