Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus
Abstract
:1. Introduction
2. Results
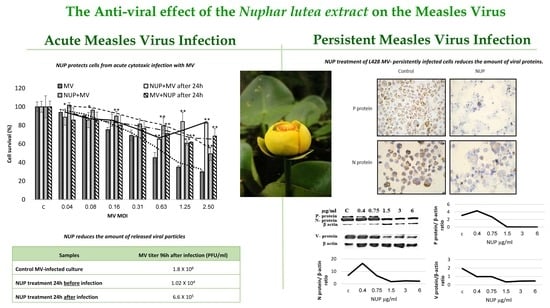
2.1. NUP Protects Cells from Acute Cytotoxic Infection with MV
2.2. NUP Treatment Reduces the Amount of Released Viral Particles
2.3. NUP Treatment of L428 MV- Persistently Infected Cells Reduces the Amount Of Viral Protein
2.4. MV RNA Expression is not Affected by NUP Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Viruses
4.2. Nuphar Lutea Extracts (NUP)
4.3. MV Acute Infection with the MV Vaccine Edmonston Strain
4.4. Western Blot Analysis
4.5. Immunohistochemistry
4.6. Infective Virus Release (PFU/mL) from NUP-Treated Cells
4.7. MV RNA Determination by Real-Time RT-PCR (qRT-PCR)
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Beyrouthy, M.; Arnold, N.; Delelis-Dusollier, A.; Dupont, F. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar] [PubMed]
- Nakae, H.; Yokoi, A.; Kodama, H.; Horikawa, A. Comparison of the Effects on Rib Fracture between the Traditional Japanese Medicine Jidabokuippo and Nonsteroidal Anti-Inflammatory Drugs: A Randomized Controlled Trial. Evid. Based. Complement. Alternat. Med. 2012, 2012, 837958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.M. Gitksan medicinal plants--cultural choice and efficacy. J. Ethnobiol. Ethnomed. 2006, 2, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uprety, Y.; Lacasse, A.; Asselin, H. Traditional Uses of Medicinal Plants from the Canadian Boreal Forest for the Management of Chronic Pain Syndromes. Pain Pract. 2016, 16, 459–466. [Google Scholar] [CrossRef]
- Padgett, D.J. A monograph of Nuphar (Nymphaeaceae). Rhodora 2007, 109, 1–95. [Google Scholar] [CrossRef]
- Ozer, J.; Levi, T.; Golan-Goldhirsh, A.; Gopas, J. Anti-inflammatory effect of a Nuphar lutea partially purified leaf extract in murine models of septic shock. J. Ethnopharmacol. 2015, 161, 86–91. [Google Scholar] [CrossRef]
- Yildirim, A.B.; Karakas, F.P.; Turker, A.U. In vitro antibacterial and antitumor activities of some medicinal plant extracts, growing in Turkey. Asian Pac. J. Trop. Med. 2013, 6, 616–624. [Google Scholar] [CrossRef]
- Okamura, S.; Nishiyama, E.; Yamazaki, T.; Otsuka, N.; Taniguchi, S.; Ogawa, W.; Hatano, T.; Tsuchiya, T.; Kuroda, T. Action mechanism of 6, 6′-dihydroxythiobinupharidine from Nuphar japonicum, which showed anti-MRSA and anti-VRE activities. Biochim. Biophys. Acta (BBA)-General Subj. 2015, 1850, 1245–1252. [Google Scholar] [CrossRef]
- Cullen, W.P.; LaLonde, R.T.; Wang, C.J.; Wong, C.F. Isolation and in vitro antifungal activity of 6, 6′-dihydroxythiobinupharidine. J. Pharm. Sci. 1973, 62, 826–827. [Google Scholar] [CrossRef]
- El-On, J.; Ozer, L.; Gopas, J.; Sneir, R.; Golan-Goldhirsh, A. Nuphar lutea: In vitro anti-leishmanial activity against Leishmania major promastigotes and amastigotes. Phytomedicine 2009, 16, 788–792. [Google Scholar] [CrossRef]
- Ozer, L.; El-On, J.; Golan-Goldhirsh, A.; Gopas, J. Leishmania major: Anti-leishmanial activity of Nuphar lutea extract mediated by the activation of transcription factor NF-κB. Exp. Parasitol. 2010, 126, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jacob, M.; Walker, L.; Tekwani, B. Screening North American plant extracts in vitro against Trypanosoma brucei for discovery of new antitrypanosomal drug leads. BMC Complement. Altern. Med. 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Yoshida, K.; Miyagawa, K.; Nemoto, Y.; Asao, Y.; Yoshikawa, M. Nuphar alkaloids with immediately apoptosis-inducing activity from Nuphar pumilum and their structural requirements for the activity. Bioorg. Med. Chem. Lett. 2006, 16, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Ozer, J.; Fishman, D.; Eilam, B.; Golan-Goldhirsh, A.; Gopas, J. Anti-metastatic effect of semi-purified Nuphar lutea leaf extracts. J. Cancer 2017, 8, 1433–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozer, J.; Eisner, N.; Ostrozhenkova, E.; Bacher, A.; Eisenreich, W.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lutea thioalkaloids inhibit the nuclear factor κappaB pathway, potentiate apoptosis and are synergistic with cisplatin and etoposide. Cancer Biol. Ther. 2009, 8, 1860–1868. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, P.; Jankovic, D.; Muscat, M.; Ben-Mamou, M.; Reef, S.; Papania, M.; Singh, S.; Kaloumenos, T.; Butler, R.; Datta, S. Measles and rubella elimination in the WHO Region for Europe: Progress and challenges. Clin. Microbiol. Infect. 2017, 23, 504–510. [Google Scholar] [CrossRef]
- Moss, W.J.; Griffin, D.E. Measles. Lancet 2012, 379, 153–164. [Google Scholar] [CrossRef]
- Griffin, D.E.; Lin, W.-H.; Pan, C.-H. Measles virus, immune control, and persistence. FEMS Microbiol. Rev. 2012, 36, 649–662. [Google Scholar] [CrossRef]
- Yoon, J.-J.; Chawla, D.; Paal, T.; Ndungu, M.; Du, Y.; Kurtkaya, S.; Sun, A.; Snyder, J.P.; Plemper, R.K. High-Throughput Screening—Based Identification of Paramyxovirus Inhibitors. J. Biomol. Screen. 2008, 13, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Barnard, D.L. Inhibitors of measles virus. Antivir. Chem. Chemother. 2004, 15, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Shook, B.C.; Lin, K. Recent advances in developing antiviral therapies for respiratory syncytial virus. Top. Curr. Chem. 2017, 375, 40. [Google Scholar] [CrossRef] [PubMed]
- Paal, T.; Brindley, M.A.; Clair, C.S.; Prussia, A.; Gaus, D.; Krumm, S.A.; Snyder, J.P.; Plemper, R.K. Probing the spatial organization of measles virus fusion complexes. J. Virol. 2009, 83, 10480–10493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiguchi, T.; Fukuda, Y.; Matsuoka, R.; Kuroda, D.; Kubota, M.; Shirogane, Y.; Watanabe, S.; Tsumoto, K.; Kohda, D.; Plemper, R.K. Structures of the prefusion form of measles virus fusion protein in complex with inhibitors. Proc. Natl. Acad. Sci. USA 2018, 115, 2496–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, M.K.; Jordan, P.C.; Stevens, S.; Tam, Y.; Deval, J.; Nichol, S.T.; Spiropoulou, C.F. Susceptibility of paramyxoviruses and filoviruses to inhibition by 2′-monofluoro-and 2′-difluoro-4′-azidocytidine analogs. Antivir. Res. 2018, 153, 101–113. [Google Scholar] [CrossRef]
- Sun, A.; Yoon, J.-J.; Yin, Y.; Prussia, A.; Yang, Y.; Min, J.; Plemper, R.K.; Snyder, J.P. Potent Non-Nucleoside Inhibitors of the Measles Virus RNA-Dependent RNA Polymerase Complex. J. Med. Chem. 2008, 51, 3731–3741. [Google Scholar] [CrossRef] [Green Version]
- Trottier, C.; Chabot, S.; Mann, K.K.; Colombo, M.; Chatterjee, A.; Miller, W.H., Jr; Ward, B.J. Retinoids inhibit measles virus in vitro via nuclear retinoid receptor signaling pathways. Antivir. Res. 2008, 80, 45–53. [Google Scholar] [CrossRef]
- White, L.K.; Yoon, J.-J.; Lee, J.K.; Sun, A.; Du, Y.; Fu, H.; Snyder, J.P.; Plemper, R.K. Nonnucleoside inhibitor of measles virus RNA-dependent RNA polymerase complex activity. Antimicrob. Agents Chemother. 2007, 51, 2293–2303. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-J.; Krumm, S.A.; Ndungu, J.M.; Hoffman, V.; Bankamp, B.; Rota, P.A.; Sun, A.; Snyder, J.P.; Plemper, R.K. Target analysis of the experimental measles therapeutic AS-136A. Antimicrob. Agents Chemother. 2009, 53, 3860–3870. [Google Scholar] [CrossRef] [Green Version]
- Dalvie, E.D.; Gopas, J.; Golan-Goldhirsh, A.; Osheroff, N. 6,6′-Dihydroxythiobinupharidine as a poison of human type II topoisomerases. Bioorg. Med. Chem. Lett. 2019, 29, 1881–1885. [Google Scholar] [CrossRef]
- Levy, D.H.; Chapple, I.L.C.; Shapira, L.; Golan-Goldhirsh, A.; Gopas, J.; Polak, D. Nupharidine enhances Aggregatibacter actinomycetemcomitans clearance by priming neutrophils and augmenting their effector functions. J. Clin. Periodontol. 2019, 46, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Tada, N.; Jansen, D.J.; Mower, M.P.; Blewett, M.M.; Umotoy, J.C.; Cravatt, B.F.; Wolan, D.W.; Shenvi, R.A. Synthesis and Sulfur Electrophilicity of the Nuphar Thiaspirane Pharmacophore. ACS Cent. Sci. 2016, 2, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Sellin, C.I.; Horvat, B. Current animal models: Transgenic animal models for the study of measles pathogenesis. Curr. Top. Microbiol. Immunol. 2009, 330, 111–127. [Google Scholar] [PubMed]
- Kis, L.L.; Nagy, N.; Klein, G.; Klein, E. Expression of SH2D1A in five classical Hodgkin’s disease-derived cell lines. Int. J. cancer 2003, 104, 658–661. [Google Scholar] [CrossRef]
- Naaman, H.; Rall, G.; Matullo, C.; Veksler-Lublinsky, I.; Shemer-Avni, Y.; Gopas, J. MiRNA-124 is a link between measles virus persistent infection and cell division of human neuroblastoma cells. PLoS ONE 2017, 12, e0187077. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Ono, N.; Tatsuo, H.; Minagawa, H.; Takeda, M.; Takeuchi, K.; Yanagi, Y. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 2002, 76, 6743–6749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Yamaguchi, S.; Matsuda, H.; Kohda, Y.; Ishikawa, H.; Tanaka, N.; Yamahara, J.; Murakami, N. Crude drugs from aquatic plants. IV. On the constituents of alismatis rhizoma. (2). Stereostructures of bioactive sesquiterpenes, alismol, alismoxide, orientalols A, B, and C, from Chinese alismatis rhizoma. Chem. Pharm. Bull. 1994, 42, 1813–1816. [Google Scholar] [CrossRef] [Green Version]
- Techaarpornkul, S.; Barretto, N.; Peeples, M.E. Functional Analysis of Recombinant Respiratory Syncytial Virus Deletion Mutants Lacking the Small Hydrophobic and/or Attachment Glycoprotein Gene. J. Virol. 2001, 75, 6825–6834. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compound Nuphar extract is available from the authors. |






| Samples | MV Titer 96 h after Infection (PFU/mL) |
|---|---|
| Control MV-infected culture | 1.8 × 108 |
| NUP treatment 24 h before infection | 1.02 × 104 |
| NUP treatment 24 h after infection | 6.6 × 105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winer, H.; Ozer, J.; Shemer, Y.; Reichenstein, I.; Eilam-Frenkel, B.; Benharroch, D.; Golan-Goldhirsh, A.; Gopas, J. Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus. Molecules 2020, 25, 1657. https://doi.org/10.3390/molecules25071657
Winer H, Ozer J, Shemer Y, Reichenstein I, Eilam-Frenkel B, Benharroch D, Golan-Goldhirsh A, Gopas J. Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus. Molecules. 2020; 25(7):1657. https://doi.org/10.3390/molecules25071657
Chicago/Turabian StyleWiner, Hila, Janet Ozer, Yonat Shemer, Irit Reichenstein, Brit Eilam-Frenkel, Daniel Benharroch, Avi Golan-Goldhirsh, and Jacob Gopas. 2020. "Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus" Molecules 25, no. 7: 1657. https://doi.org/10.3390/molecules25071657