Synthesis, Structural, and Cytotoxic Properties of New Water-Soluble Copper(II) Complexes Based on 2,9-Dimethyl-1,10-Phenanthroline and Their One Derivative Containing 1,3,5-Triaza-7-Phosphaadamantane-7-Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
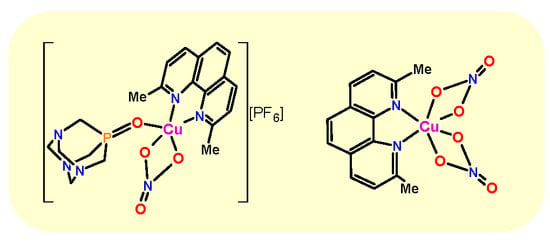
2.2. Crystal Structures
2.3. Magnetic Properties
2.4. Cytotoxic Assays
2.5. Apo-Transferrin Interactions
3. Experimental
3.1. Materials and Methods
3.2. Cell Cultures
3.3. Cytotoxic Properties on Normal and Cancer Cell Lines - Quantitative Suspension Test According to EN 14476
3.4. Apo-Transferrin Interactions
3.4.1. Fluorescence Spectroscopy
3.4.2. CD Spectroscopy
3.5. Synthesis and Analytical Data
3.6. Stability/Solubility Tests and Octanol-Water Partition Coefficient Determination
3.7. X-ray Crystallography
3.8. Magnetic Measurement
3.9. EPR Spectra
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 978-0-470-51234-0. [Google Scholar]
- MacGillivray, L.R. Metal-Organic Frameworks: Design and Applications, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-0-470-19556-7. [Google Scholar]
- Balakrishna, M.S. Copper(I) Chemistry of Phosphines, Functionalized Phosphines and Phosphorus Heterocycles; Elsevier: Waltham, MA, USA, 2019. [Google Scholar]
- Zhang, Q.-C.; Xiao, H.; Zhang, X.; Xu, L.-J.; Chen, Z.-N. Luminescent oligonuclear metal complexes and the use in organic light-emitting diodes. Coord. Chem. Rev. 2019, 378, 121–133. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Di, D.; Romanov, A.S.; Yang, L.; Richter, J.M.; Rivett, J.P.; Jones, S.; Thomas, T.H.; Abdi Jalebi, M.; Friend, R.H.; Linnolahti, M.; et al. High-performance light-emitting diodes based on carbene-metal-amides. Science 2017, 356, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, M.; Hoshino, M. Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Yersin, H., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp. 119–171. ISBN 978-3-527-69175-3. [Google Scholar]
- Thompson, L.K.; Dawe, L.N. Magnetic properties of transition metal (Mn(II), Mn(III), Ni(II), Cu(II)) and lanthanide (Gd(III), Dy(III), Tb(III), Eu(III), Ho(III), Yb(III)) clusters and [nxn] grids: Isotopic exchange and SMM behavior. Coord. Chem. Rev. 2015, 289–290, 13–31. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C-H bonds oxidative functionalization: Recent advances. Coord Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Śliwa, E.I.; Nesterov, D.S.; Kłak, J.; Jakimowicz, P.; Kirillov, A.M.; Smoleński, P. Unique Copper−Organic Networks Self-Assembled from 1,3,5-Triaza-7-Phosphaadamantane and Its Oxide: Synthesis, Structural Features, and Magnetic and Catalytic Properties. Cryst. Growth Des. 2018, 18, 2814–2823. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Di Nicola, C.; Tombesi, A.; Pettinari, R. Coordination chemistry of pyrazolone-based ligands and applications of their metal complexes. Coord. Chem. Rev. 2019, 401, 213069–213146. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Chapter One—Homogeneous Multicopper Catalysts for Oxidation and Hydrocarboxylation of Alkanes. Adv. Inorg. Chem. 2013, 65, 1–31. [Google Scholar] [CrossRef]
- Gao, X.; Xu, T.; Jiang, Z.; Yu, H.; Wang, Y.; He, Y. Rational construction and remarkable gas adsorption properties of a HKUST-1-like tbo-type MOF based on a tetraisophthalate linker. Dalton Trans. 2019, 48, 16793–16799. [Google Scholar] [CrossRef]
- Gooneratne, S.R.; Buckley, W.T.; Christensen, D.A. Review of copper deficiency and metabolism in ruminants. Can. J. Anim. Sci. 1989, 69, 819–845. [Google Scholar] [CrossRef]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and biomedical applications of copper sulfide nanoparticles: From sensors to theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Duff, B.; Thangella, V.R.; Creaven, B.S.; Walsh, M.; Egan, D.A. Anti-cancer activity and mutagenic potential of novel copper(II) quinolinone Schiff base complexes in hepatocarcinoma cells. Eur. J. Pharm. 2012, 689, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, S.; Fathima, A.; Rao, J.R.; Nair, B.U. Antibacterial activity of copper(II) complexes against Staphylococcus aureus. RSC Adv. 2014, 4, 32004–32012. [Google Scholar] [CrossRef]
- Teguh, H.S.; Fahimah, M. Synthesis of metal-organic (complexes) compounds copper(II)-imidazole for antiviral HIV candidate. Ind. J. Trop. Infect. Dis. 2016, 6, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of copper-binding proteins in breast cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA): Transition metal complexes and related catalytic, medicinal and photoluminescent applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Bravo, J.; Bolaño, J.; Gonsalvi, L.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA) and derivatives. Part II. The quwst for tailored ligands, complexes and related applications. Coord. Chem. Rev. 2009, 254, 555–607. [Google Scholar] [CrossRef]
- Guerriero, A.; Peruzzini, M.; Gonsalvi, L. Coordination chemistry of 1,3,5-triaza-7-phosphatricyclo [3.3.1.1]decane (PTA) and derivatives. Part III. Variations on theme: Novel architectures, materials and applications. Coord. Chem. Rev. 2018, 355, 328–361. [Google Scholar] [CrossRef]
- Frost, B.J.; Harkreader, J.L.; Bautista, C.M. Synthesis and solid state structure of Co(II) complexes of O=PTA. Inorg. Chem. Commun. 2008, 11, 580–583. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Smoleński, P.; Kuznetsov, M.L.; Pombeiro, A.J.L. Oxorhenium complexes bearing the water-soluble tris(pyrazol-1-yl)methanesulfonate, 1,3,5-triaza-7-phosphaadamantane or related ligands, as catalysts for Baeyer-Villiger oxidation of ketones. Inorg. Chem. 2013, 52, 4534–4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoleński, P.; Kochel, A. Synthesis of the first Monodentate S- and O-Coordinating 1,3,5-Triaza-7-phosphaadamantane-7-chalcogenides [CoCl(bpy)2(Z-PTA=Z)]X (Z=S, O; bpy = 2,2′-bipyridine; X=BF4, PF6) and [CoCl(bpy)2(N-PTA)]BF4 (PTA = 1,3,5-Triaza-7-phosphaadamantane). Polyhedron 2010, 29, 1561–1566. [Google Scholar] [CrossRef]
- Śliwa, E.I. Synthesis and structural studies of the new copper(I/II) complexes with organic (P,N) ligands. Master’s Thesis, University of Wrocław, Wrocław, Poland, 2014. [Google Scholar]
- Jaremko, L.; Kirillov, A.M.; Smoleński, P.; Lis, T.; Pombeiro, A.J.L. Extending the Coordination Chemistry of 1,3,5-Triaza-7-phosphaadamantane (PTA) to Cobalt Centres: First Examples of Co-PTA complexes and of a Metal Complex with the PTA Oxide Ligand. Inorg. Chem. 2008, 47, 2922–2924. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Wieczorek, S.W.; Lis, A.; Guedes da Silva, M.F.C.; Florek, M.; Król, J.; Staroniewicz, Z.; Smoleński, P.; Pombeiro, A.J.L. 1,3,5-triaza-7-phosphaadamantane-7-oxide (PTA=O): Nwe diamondoid building block for design of three-dimensional metal-organic frameworks. Cryst. Growth Des. 2011, 11, 2711–2716. [Google Scholar] [CrossRef]
- Rancan, M.; Tessarolo, J.; Casarin, M.; Zanonato, P.L.; Quici, S.; Armelao, L. Double Level Selection in a Constitutional Dynamic Library of Coordination Driven Supramolecular Polygons. Inorg. Chem. 2014, 53, 7276–7287. [Google Scholar] [CrossRef]
- Rancan, M.; Tessarolo, J.; Quici, S.; Armelao, L. Post-assembly guest oxidation in a metallo-supramolecular host and structural rearrangement to a coordination polymer. Chem. Commun. 2014, 50, 13761–13764. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Guedes da Silva, M.F.C.; Śliwa, E.I.; Smolenński, P.; Kuznetsov, M.L.; Pombeiro, A.J.L. Copper(II) and Sodium(I) Complexes based on 3,7-Diacetyl-1,3,7-triaza-5 phosphabicyclo [3.3.1]nonane-5-oxide: Synthesis, Characterization, and Catalytic Activity. Chem. Asian J. 2018, 13, 2868–2880. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Solim, M.E. Metal complexes in cancer therapy - an update from drug design perspective. Drug Des. Devel. 2017, 11, 599–616. [Google Scholar] [CrossRef] [Green Version]
- Smoleński, P.; Jaros, S.W.; Pettinari, C.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Kochel, A.; Kirillov, A.M. New Water-soluble polypyridine silver(I) derivatives of 1,3,5-Triaza-7-phosphaadamantane (PTA) with significant antimicrobial and antiproliferative activity. Dalton Trans. 2013, 42, 6572–6581. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Smoleński, P.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Fernandes, A.R.; Luis, D.; Silva, A.; Santos, S.; Borralho, P.M.; Rodrigues, C.M.P.; et al. Cobalt and Zinc Compounds Bearing 1,10-phenanthroline-5,6-dione or 1,3,5-tiaza-7-phosphaadamantane Derivatives – Synthesis, Characterization, Cytotoxicity, and Cell Selectivity Studies. Eur. J. Inorg. Chem. 2013, 3651–3658. [Google Scholar] [CrossRef] [Green Version]
- Wołoszyn, A.; Pettinari, C.; Pettinari, R.; Badillo Patzmay, G.V.; Kwiecień, A.; Lupidi, G.; Nabissi, M.; Santoni, G.; Smolenński, P. Ru(II)-(PTA) and –mPTA complexes with N2-donor ligands bipyridyl and phenanthroline and their antiproliferative activities on human multiple myeloma cell lines. Dalton Trans. 2017, 46, 10073–10081. [Google Scholar] [CrossRef] [Green Version]
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiów, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper(I) complexes with phosphines P(p-OCH3-Ph)2CH2OH and P(p-OCH3-Ph)2CH2SarGly: Synthesis, multimodal DNA interactions, and prooxidative and in vitro antiproliferative activity. J. Inorg. Biochem. 2020, 203, 110926/1–110926/14. [Google Scholar] [CrossRef]
- Ng, N.S.; Wu, M.J.; Aldrich-Wright, J.R. The cytotoxicity of some phenanthroline-based antimicrobial copper(II) and ruthenium(II) complexes. J. Inorg. Biochem. 2018, 180, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.M.; Smoleński, P.; Haukka, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Unprecedented Metal-free C(sp3)–C(sp3) Bond Cleavage: Switching from N-alkyl- to N-methyl-1,3,5-triaza-7-phosphaadamantane. Organometallics 2009, 28, 1683–1687. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Kirillov, A.M.; Smoleński, P.; Pombeiro, A.J.L. Engineering Coordination and Supramolecular Copper-organic Networks by Aqueous Medium Self-assembly with 1,3,5-Triaza-7-phosphaadamantane (PTA). Cryst. Growth Des. 2009, 9, 3006–3010. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Smoleński, P.; Ma, Z.; Guedes da Silva, M.F.C.; Haukka, M.; Pombeiro, A.J.L. Copper(I) Iodide Complexes derived from N-alkyl-1,3,5-Triaza-7-phosphaadamantanes: Synthesis, Crystal Structures, Photoluminescence and Identification of the Unprecedented {Cu3I5}2-Cluster. Organometallics 2009, 28, 6425–6431. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Filipowicz, M.; Guedes da Silva, M.F.C.; Kłak, J.; Smoleński, P.; Pombeiro, A.J.L. Unprecedented Mixed-Valence Cu(I)/Cu(II) Complex Derived from N-Methyl-1,3,5-triaza-7-phosphaadamantane: Synthesis, Structural Features and Magnetic Properties. Organometallics 2012, 31, 7921–7925. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Guedes da Silva, M.F.C.; Sokolnicki, J.; Smolenński, P.; Pombeiro, A.J.L. Hydrosoluble Cu(I)-DAPTA complexes: Synthesis, characterization, luminescence thermochromism and catalytic activity for microwave-assisted three-component azide-alkyne cycloaddition click reaction. Dalton Trans. 2018, 47, 7290–7299. [Google Scholar] [CrossRef]
- Smoleński, P.; Kłak, J.; Nesterov, D.S.; Kirillov, A.M. Unique Mixed-Valence Cu(I)/Cu(II) Coordination Polymer with New Topology of Bitubular 1D Chains Driven by 1,3,5-Triaza-7-phosphaadamantane (PTA). Cryst. Growth Des. 2012, 12, 5852–5857. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Smoleński, P.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. The First Copper Complexes Bearing the 1,3,5-Triaza-7-phosphaadamantane (PTA) Ligand. Eur. J. Inorg. Chem. 2007, 2686–2692. [Google Scholar] [CrossRef]
- Wanke, R.; Smoleński, P.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Cu(I) complexes bearing the new sterically demanding and coordination flexible tris(3-phenyl-1-pyrazolyl)methanesulfonate (TpmsPh) ligand and the water-soluble phosphine 1,3,5-triaza-7-phosphaadamantane (PTA) or related ligands. Inorg. Chem. 2008, 47, 10158–10168. [Google Scholar] [CrossRef]
- Jaros, S.W.; Sokolnicki, J.; Wołoszyn, A.; Haukka, M.; Kirillov, A.M.; Smoleński, P. Novel 2D coordination network built from hexacopper(I)-iodide clusters and cagelike aminophosphine blocks for reversible “turn-on” sensing of aniline. J. Mater. Chem. C 2018, 6, 1670–1678. [Google Scholar] [CrossRef]
- Leandri, V.; Daniel, Q.; Chen, H.; Sun, L.; Gardner, J.M.; Kloo, L. Electronic and Structural Effects of Inner Sphere Coordination of Chloride to a Homoleptic Copper(II) Diimine Complex. Inorg. Chem. 2018, 57, 4556–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaros, S.W.; Smoleński, P.; Guedes da Silva, F.C.; Florek, M.; Król, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M.; Guedes da Silva, F.C.; Florek, M.; et al. New silver BioMOFs driven by 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTA=S): Synthesis, topological analysis and antimicrobial activity. Cryst. Eng. Comm. 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-471-74493-1. [Google Scholar]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra? Academic Press: San Diego, CA, USA, 2001; Volume 1–2, ISBN 0-12-523475-9. [Google Scholar]
- Frost, B.J.; Lee, W.-C.; Pal, K.; Kim, T.H.; VanDerveer, D.; Rabinovich, D. Synthesis, structure, and coordination chemistry of O=PTA and S=PTA with 12 metals (PTA = 1,3,5-triaza-7-phosphaadamantane). Polyhedron 2010, 29, 2373–2380. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donors ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Oliveira, W.X.C.; Pereira, C.L.M.; Pinheiro, C.B.; Lloret, F.; Julve, M. Oxotris(oxalate)niobate(V): An oxalate delivery agent in the design of building blocks. J. Coord. Chem. 2018, 71, 707–724. [Google Scholar] [CrossRef]
- Ding, C.-F.; Li, X.-M.; Zhu, M.; Xu, H.; Zhang, S.-S. Aquadichloro(2,9-dimethyl-1,10-phenanthroline-ĸ2N,N)copper(II). Acta Cryst. Sect. E Struct. Rep. Online 2006, 62, m604–m605. [Google Scholar] [CrossRef]
- Zhai, C.-P.; Yan, F.-M.; Zhao, P.-Z. (2,9-Dimethyl-1,10-phenaanthroline-ĸ2N,N’)(4-hydroxybenzoato-ĸ2O,O’)-(nitrato-ĸO)copper(II). Acta Cryst. Sect. E Struct. Rep. Online 2008, 64, m1479. [Google Scholar] [CrossRef] [Green Version]
- Marsh, R.E. The perils of Cc revisited. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 317–322. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Weyhermuller, T.; Freddy, A.J.; Vasanthi, H.R.; Nair, B.U. Anomalous behavior of pentacoordinate copper complexes of dimethylphenanthroline and derivatives of terpyridine ligands: Studies on DNA binding, cleavage and apoptotic activity. Eur. J. Med. Chem. 2011, 46, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Singh, A.; Singh, A.; Singh, N. Syntheses, crystal and photophysical properties of Cu(II) complexes: Fine tuning of a coordination sphere for selective binding of azamethiphos. Dalton Trans. 2017, 46, 985–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biradha, K.; Fujita, M. Co-ordination polymer containing square grids of dimension 15 × 15 Å. Journal of the Chemical Society. Dalton Trans. 2000, 21, 3805–3810. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Weinheim, Germany, 1993; pp. 103–134. ISBN 3-527-89566-3. [Google Scholar]
- Smart, J.S. Effective Field Theories of Magnetism; W.B. Saunders Comp.: Philadelphia, PA, USA; London, UK, 1966; pp. 139–154. ISBN 978-3-642-70735-3. [Google Scholar]
- Carlin, R.L. Magnetochemistry; Springer: Berlin, Germany, 1986; pp. 52–108. ISBN1 10 1124092226. ISBN2 13 978-1124092225. [Google Scholar]
- Cristóvão, B.; Miroslaw, B.; Kłak, J. New mononuclear CuII and tetranuclear CuII2–LaIII2 Schiff base complexes—Physicochemical properties. Polyhedron 2013, 62, 218–226. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Miroslaw, B. Synthesis and characterization of CuII–LnIII (Ln = Ho, Tm, Yb or Lu) complexes with N2O4-donor Schiff base ligand. J. Coord. Chem. 2014, 67, 2728–2746. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Stefankiewicz, A.R.; Gorczyński, A.; Kubicki, M.; Kłak, J.; Korabik, M.J.; Patroniak, V. Structural, spectroscopic and magnetic properties of new copper(II) complexes with a terpyridine ligand. Polyhedron 2011, 30, 233–240. [Google Scholar] [CrossRef]
- Pilbrow, J.R. Transition Ion Electron Paramagnetic Resonance; Oxford Science Publications: Oxford, UK, 1990; pp. 57–150. ISBN1 0198552149. ISBN2 9780198552147. [Google Scholar]
- Hathaway, B.J. Copper in Comprehensive Coordination Chemistry, The synthesis, Reactions, Properties and Applications of Coordination Compounds, 1st ed.; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 5, pp. 533–774. ISBN 0-08-035948-5. [Google Scholar]
- Hathaway, B.J. The correlation of the electronic properties and stereochemistry of mononuclear {CuN4–6} chromophores. J. Chem. Soc. Dalton Trans. 1972, 1196–1199. [Google Scholar] [CrossRef]
- Elliott, H.; Hathaway, B.J.; Slade, R.C. The electronic properties of monohalogenobisbipyridyl copper(II) complexes. J. Chem. Soc. A. 1966, 1443–1445. [Google Scholar] [CrossRef]
- Procter, I.M.; Hathaway, B.J.; Nicholls, P. The electronic properties and stereochemistry of the copper(II) ion. Part, I. Bis(ethylenediamine)copper(II) complexes. J. Chem. Soc. A 1968, 1678–1984. [Google Scholar] [CrossRef]
- Jaros, S.W.; Śliwińska-Hill, U.; Białońska, A.; Nesterov, D.S.; Kuropka, P.; Sokolnicki, J.; Bażanów, B.; Smoleński, P. Light-Stable Polypyridine Silver(I) Complexes of 1,3,5-Triaza-7-Phosphaadamantane (PTA) and 1,3,5-Triaza-7-Phosphaadamantane-7-Sulfide (PTA=S): Significant Antiproliferative Activity of Representative Examples in Aqueous Media. Dalton Trans. 2019, 48, 11235–11249. [Google Scholar] [CrossRef] [PubMed]
- Kennt, R.G.; Marmion, C.J. Enhancing the Therapeutic Potential of Platinum-based Anticancer Agents by Incorporating Clinically Approved Drugs as Ligands. In Metal-Based Anticancer Agents, 1st ed.; Casini, A., Vessières, A., Meier-Menches, S.M., Eds.; RSC: London, UK, 2019; p. 23. ISBN 978-1788014069. [Google Scholar]
- Konarikova, K.; Frivaldska, J.; Gbelcova, H.; Sveda, M.; Ruml, T.; Janubova, M.; Zitnanova, I. Schiff base Cu(II) complexes as inhibitors of proteasome in human cancer cells. Bratisl. Med. J. 2019, 120, 646–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyalakshmi, K.; Selvakumaran, N.; Bhuvanesh, N.S.P.; Sreekanth, A.; Karvembu, R. DNA/protein binding and cytotoxicity studies of copper(II) complexes containing N,N’,N’’-trisubstituted guanidine ligands. RSC Adv. 2014, 4, 17179–17195. [Google Scholar] [CrossRef]
- Ganeshpandian, M.; Ramakrishnan, S.; Palaniandavar, M.; Suresh, E.; Riyasdeen, A.; Akbarsha, M.A. Mixed ligand copper(II) complexes of 2,9-dimethyl-1,10-phenanthroline: Tridentate 3N primary ligands determine DNA binding and cleavage and cytotoxicity. J. Inorg. Biochem. 2014, 140, 202–212. [Google Scholar] [CrossRef]
- García-Moreno, E.; Gascón, S.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. S-Propargylthiopyridine Phosphane Derivatives as Anticancer Agents: Characterization and Antitumor Activity. Organometallics 2013, 32, 3710–3720. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. In Vitro Anticancer Activity of Cis-Diammineplatinum(II) Complexes with B-Diketonate Leaving Group Ligands. J. Med. Chem. 2012, 55, 5326–5336. [Google Scholar] [CrossRef] [Green Version]
- Marouzi, S.; Sharifi Rad, A.; Beigoli, S.; Teimoori Baghaee, P.; Assaran Darban, R.; Chamani, J. Study on effect of lomefloxacin on human holo-transferrin in the presence of essential and nonessential amino acids: Spectroscopic and molecular modeling approaches. Int. J. Biol. Macromol. 2017, 97, 688–699. [Google Scholar] [CrossRef]
- Omidvar, Z.; Parivar, K.; Sanee, H.; Amiri-Tehranizadeh, Z.; Baratian, A.; Saberi, M.R.; Asoodeh, A.; Chamani, J. Investigations with spectroscopy, zeta potential and molecular modeling of the non-cooperative behaviour between cyclophosphamide hydrochloride and aspirin upon interaction with human serum albumin: Binary and ternary systems from multi-drug therapy. J. Biomol. Struct. Dyn. 2011, 29, 181–206. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, G.; Dong, Y.; Zhao, Y.Q.; Sun, X.R.; Chen, L.; Chen, H.B. Studies on the binding of fulvic acid with transferrin by spectroscopic analysis. Spectrochim. Acta Part. A 2015, 137, 1280–1285. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Fluorescence Studies on PAMAM Dendrimers Interactions with Bovine Serum Albumin. Bioelectrochemistry 2002, 55, 33–35. [Google Scholar] [CrossRef]
- Ware, W.R. Oxygen Quenching of Fluorescence in Solution: An Experimental Study of the Diffusion Process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Groessl, M.; Terenghi, M.; Casinia, A.; Elvirib, L.; Lobinski, R.; Dyson, P.J. Reactivity of anticancer metallodrugs with serum proteins: New insights from size exclusion chromatography-ICP-MS and ESIMS. Anal. Spectrom. 2010, 25, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnev, A.V.; Aleksenko, S.S.; Semenova, O.; Hartinger, C.G.; Timerbaev, A.R.; Keppler, B.K. Determination of binding constants and stoichiometries for platinum anticancer drugs and serum transport proteins by capillary electrophoresis using the Hummel-Dreyer method. J. Sep. Sci. 2005, 28, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.F.; Toneatto, J.; Silvero, M.J.; Argüello, G.A. Binding of [Cr(phen)3]3+ to transferrin at extracellular and endosomal pHs: Potential application in photodynamic therapy. Biochim. Biophys. Acta 2014, 1840, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, O.; Kurpiewska, K.; Lewiński, K.; Stochel, G.; Brindell, M. Interaction of apo-transferrin with anticancer ruthenium complexes NAMI-A and its reduced form. J. Inorg. Biochem. 2012, 116, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Leckband, D. Measuring the Forces That Control Protein Interactions. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daigle, D.J.; Pepperman, A.B., Jr.; Vail, S.L. Synthesis of a monophosphorus analog of hexamethylenetetramine. J. Heterocycl. Chem. 1974, 11, 407–408. [Google Scholar] [CrossRef]
- Daigle, D.J. 1,3,5-Triaza-7-Phosphatricyclo[3.3.1.13,7]Decane and Derivatives. Inorg. Synth 1998, 32, 40–45. [Google Scholar] [CrossRef]
- EN 14476. Chemical Disinfectants and Antiseptics – Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area—Test Method and Requirements (Phase2/Step 1); European Committee for Standarization: Brussels, Belgium, 2013. [Google Scholar]
- Sangster, J. Octanol-water partition coefficients of simple organic compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1227. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]









| Formula Unit | [C20H24CuN6O4P][PF6] (1) | [C14H12CuN4O6][C2H3N] (3) |
|---|---|---|
| Formula weight | 651.93 | 436.87 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | I2/a |
| a (Å) | 10.094(3) | 10.500(7) |
| b (Å) | 17.125(4) | 14.480(8) |
| c (Å) | 14.330(4) | 12.426(9) |
| β (°) | 95.44(3) | 106.71(5) |
| Z | 4 | 4 |
| Volume (Å3) | 2465.9(12) | 1809(2) |
| T (K) | 100(2) | 100(2) |
| Dc (g cm−3) | 1.756 | 1.604 |
| μ (mm−1) | 1.103 | 1.253 |
| θmax, θmin (°) | 36.95, 3.50 | 28.69, 3.58 |
| Rfls. measure, independent | 39426, 11361 | 6557, 2183 |
| Rint | 0.0539 | 0.0574 |
| R1 a,wR2 b [I > 2σ(I)] | 0.0442, 0.1195 | 0.0530, 0.1365 |
| R1, wR2 (all data) | 0.0526, 0.1268 | 0.0604, 0.1548 |
| Cell Line | 1 | 2 | 3 | Cu(NO3)2 | PTA=O | Dmphen | Cisplatin |
|---|---|---|---|---|---|---|---|
| NHDF | 0.57 ± 0.08 | 0.23 ± 0.03 | 1.72 ± 0.25 | 310 ± 47 | nd | nd | 16.6 ± 2.1 [74] |
| A549 | 0.29 ± 0.01 | 0.28 ± 0.04 | 0.43 ± 0.06 | 155 ± 23 | nd | nd | 33.3 ± 4.2 [74] |
| HeLa | 1.12 ± 0.16 | 1.13 ± 0.17 | 0.43 ± 0.06 | 19.1 ± 2.9 | nd | 720 ± 108 | 16.6 ± 3.1 [74] |
| MCF7 | 0.57 ± 0.08 | 0.57 ± 0.08 | 3.45 ± 0.51 | 155 ± 23 | nd | nd | 33.3 ± 4.2 [74] |
| LoVo | 0.57 ± 0.08 | 1.13 ± 0.17 | 1.72 ± 0.25 | 38.8 ± 5.8 | nd | 360 ± 54 | 9.12 ± 0.005 [75] |
| Parameter | Complex | 300K | 310K |
|---|---|---|---|
| Ksv·104 [M−1] | 1 | 2.50 ± 0.011 | 2.92 ± 0.029 |
| 2 | 4.03 ± 0.050 | 5.69 ± 0.024 | |
| 3 | 5.37 ± 0.023 | 3.51 ± 0.062 | |
| Kq·1013 [M−1s−1] | 1 | 1.00 ± 0.011 | 1.17 ± 0.029 |
| 2 | 1.61 ± 0.050 | 2.27 ± 0.024 | |
| 3 | 0.12 ± 0.023 | 1.40 ± 0.062 |
| Parameter | Complex | 300K | 310K |
|---|---|---|---|
| Ka·106 [M−1] | 1 | 1.03 ± 0.16 | 0.843 ± 0.17 |
| 2 | 4.38 ± 0.24 | 3.04 ± 0.32 | |
| 3 | 9.26 ± 0.37 | 9.38 ± 0.33 | |
| n | 1 | 1.26 ± 0.033 | 1.24 ± 0.036 |
| 2 | 1.35 ± 0.050 | 1.31 ± 0.066 | |
| 3 | 1.40 ± 0.076 | 1.42 ± 0.068 |
| T [K] | H0 [kJ mol−1] | S0 [J mol−1 K−1] | G0 [kJ mol−1] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| 300 | −15.33 | −28.24 | 962.34 | 63.99 | 33.02 | 136.57 | −34.53 | −38.15 | −40.01 |
| 310 | −35.17 | −38.45 | −41.37 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwa, E.I.; Śliwińska-Hill, U.; Bażanów, B.; Siczek, M.; Kłak, J.; Smoleński, P. Synthesis, Structural, and Cytotoxic Properties of New Water-Soluble Copper(II) Complexes Based on 2,9-Dimethyl-1,10-Phenanthroline and Their One Derivative Containing 1,3,5-Triaza-7-Phosphaadamantane-7-Oxide. Molecules 2020, 25, 741. https://doi.org/10.3390/molecules25030741
Śliwa EI, Śliwińska-Hill U, Bażanów B, Siczek M, Kłak J, Smoleński P. Synthesis, Structural, and Cytotoxic Properties of New Water-Soluble Copper(II) Complexes Based on 2,9-Dimethyl-1,10-Phenanthroline and Their One Derivative Containing 1,3,5-Triaza-7-Phosphaadamantane-7-Oxide. Molecules. 2020; 25(3):741. https://doi.org/10.3390/molecules25030741
Chicago/Turabian StyleŚliwa, Ewelina I., Urszula Śliwińska-Hill, Barbara Bażanów, Miłosz Siczek, Julia Kłak, and Piotr Smoleński. 2020. "Synthesis, Structural, and Cytotoxic Properties of New Water-Soluble Copper(II) Complexes Based on 2,9-Dimethyl-1,10-Phenanthroline and Their One Derivative Containing 1,3,5-Triaza-7-Phosphaadamantane-7-Oxide" Molecules 25, no. 3: 741. https://doi.org/10.3390/molecules25030741