Synergistic Antitumor Effects on Drug-Resistant Breast Cancer of Paclitaxel/Lapatinib Composite Nanocrystals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization
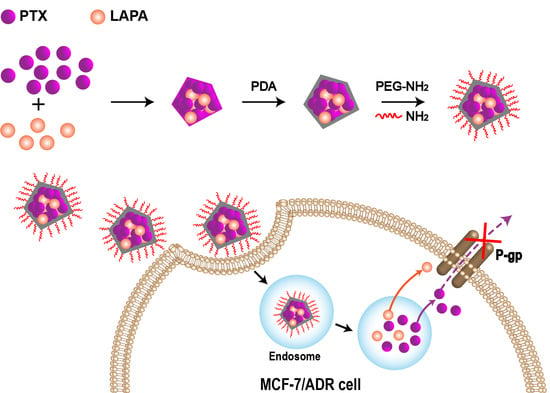
2.2. Successful Preparation of cNC@PDA-PEG
2.3. Hemolysis Assay
2.4. Enhanced Cellular Uptake and Cytotoxicity of Nanocrystals by PEG Modification
2.5. cNCs Entered Cells in an Intact Form
3. Materials and Methods
3.1. Materials
3.2. Preparation of cNC, cNC@PDA and cNC@PDA-PEG
3.3. Characterization
3.3.1. Particle Size and Distribution
3.3.2. Morphology
3.3.3. Drug Loading (DL) and Encapsulation Efficiency (EE)
3.3.4. X-ray Powder Diffraction (XRPD) and Differential Scanning Calorimeter (DSC)
3.3.5. Stability
3.3.6. In Vitro Release
3.3.7. Hemolysis Assay
3.4. Cellular Experiments
3.4.1. Cellular Uptake
3.4.2. In Vitro Cytotoxicity
3.4.3. AIE Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, X.J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol. Biol. 2010, 596, 467–488. [Google Scholar] [PubMed] [Green Version]
- Bock, C.; Lengauer, T. Managing drug resistance in cancer: Lessons from HIV therapy. Nat. Rev. Cancer 2012, 12, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Shen, J.N.; Zhang, Z.W.; Yu, H.J.; Li, Y.P. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Res. 2013, 65, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbutts, T.; Frewin, C.; Fox, R.M.; Tattersall, M.H. On the receiving endpatient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208. [Google Scholar] [CrossRef]
- Kavallaris, M. Microtubules and resistance to tubulin binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, Z.; Yuan, L.; Xu, S.; Yao, Z.; Qiao, L.; Li, K. Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer. Am. J. Cancer Res. 2016, 6, 2345–2350. [Google Scholar]
- Ajani, J.A.; Ilson, D.H.; Daugherty, K.; Pazdur, R.; Lynch, P.M.; Kelsen, D.P. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J. Natl. Cancer Inst. 1994, 86, 1086–1091. [Google Scholar] [CrossRef]
- Kato, K.; Tahara, M.; Hironaka, S.; Muro, K.; Takiuchi, H.; Hamamoto, Y.; Imamoto, H.; Amano, N.; Seriu, T. A phase II study of PTX biweekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum based chemotherapy. Cancer Chemother. Pharmacol. 2011, 67, 1265–1272. [Google Scholar] [CrossRef]
- Polee, M.B.; Eskens, F.A.; Van der Burg, M.E.; Splinter, T.A.; Siersema, P.D.; Tilanus, H.W.; Verweij, J.; Stoter, G.; van der Gaast, A. Phase II study of bi-weekly administration of paclitaxel and cisplatin in patients with advanced oesophageal cancer. Br. J. Cancer 2002, 86, 669–673. [Google Scholar] [CrossRef] [Green Version]
- Kirtane, A.R.; Kalscheuer, S.M.; Panyam, J. Exploiting nanotechnology to overcome tumor drug resistance: challenges and opportunities. Adv. Drug Deliv. Rev. 2013, 65, 1731–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powles, T.; Huddart, R.A.; Elliott, T.; Sarker, S.J.; Ackerman, C.; Jones, R.; Hussain, S.; Crabb, S.; Jagdev, S.; Chester, J. Phase III, doubleblind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J. Clin. Oncol. 2017, 35, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.L.; Doval, D.C.; Chavez, M.A.; Ang, P.C.; Aziz, Z.; Nag, S.; Ng, C.; Franco, S.X.; Chow, L.W.; Arbushites, M.C. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J. Clin. Oncol. 2008, 26, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Riera, K.J.; Haag, G.M.; Pohl, M.; Patience, P.T.; Bassermann, F.; Helbig, U.; Weibinger, F.; Schnoy, E.; Berker, K. Lapatinib versus lapatinib plus capecitabine as second-line treatment in human epidermal growth factor receptor 2-amplified metastatic gastro-oesophageal cancer: A randomised phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Eur. J. Cancer 2015, 51, 569–576. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Gomez, H.L. Combined lapatinib and paclitaxel in HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 2009, 6, 308–309. [Google Scholar] [CrossRef]
- Guan, Z.; Xu, B.; De Silvio, M.L.; Shen, Z.; Arpornwirat, W.; Tong, Z.; Lorvidhaya, V.; Jiang, Z.; Yang, J.; Makhson, A.; et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2013, 31, 1947. [Google Scholar] [CrossRef]
- Di Leo, A.; Gomez, H.L.; Aziz, Z.; Zvirbule, Z.; Bines, J.; Arbushites, M.C.; Guerrera, S.F.; Koehler, M.; Oliva, C.; Stein, S.H.; et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J. Clin. Oncol. 2008, 26, 5544–5552. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.; Chung, H.C.; Sun, G.; Doi, T.; Xu, J.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.; et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTANA - a randomized, phase III study. J. Clin. Oncol. 2014, 32, 2039–2089. [Google Scholar] [CrossRef]
- Zacharoula, I.; Athina, A.; Efstathia, V.; Konstantinos, A.; Constantinos, T. Star-Graft Quarterpolymer-Based Polymersomes as Nanocarriers for Co-Delivery of Hydrophilic/Hydrophobic Chemotherapeutic Agents. ACS Omega 2018, 3, 11896–11908. [Google Scholar]
- Mi, F.L.; Wang, L.F.; Chu, P.Y.; Peng, S.L.; Feng, C.L.; Lai, Y.J.; Li, J.N.; Lin, Y.H. Active Tumor-Targeted co-Delivery of Epigallocatechin Gallate and Doxorubicin in Nanoparticles for Combination Gastric Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 2847–2859. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Goldman, A.; Kulkarni, A.; Kohandel, M.; Pandey, P.; Rao, P.; Natarajan, S.K.; Sabbisetti, V.; Sengupta, S. Rationally Designed 2 in 1 Nanoparticles Can Overcome Adaptive Resistance in Cancer. ACS Nano 2016, 10, 5823–5834. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, S.; Wang, F.; Zou, A.; Zhang, S.; Xiong, Y.; Cao, S.; Zhang, Q.; Wang, Y.; Jiang, X. A novel combined micellar system of lapatinib and paclitaxel with enhanced antineoplastic effect against human epidermal growth factor receptor-2 positive breast tumor in vitro. J. Pharm. Sci. 2015, 104, 165–177. [Google Scholar] [CrossRef]
- Vergara, D.; Bellomo, C.; Zhang, X.; Vergaro, V.; Tinelli, A.; Lorusso, V.; Rinaldi, R.; Lvov, Y.M.; Leporatti, S.; Maffia, M. Lapatinib/paclitaxel polyelectrolyte nanocapsules for overcoming multidrug resistance in ovarian cancer. Nanomedicine-UK 2012, 8, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Danquah, M.; Singh, S.; Wu, H.; Mahato, R. Paclitaxel- and lapatinib-loaded lipopolymer micelles overcome multidrug resistance in prostate cancer. Drug Deliv. Transl. Res. 2011, 1, 420–428. [Google Scholar]
- Hu, H.X.; Lin, Z.Q.; He, B.; Dai, W.B.; Wang, X.Q.; Wang, J.C.; Zhang, X.; Zhang, H.; Zhang, Q. A novel localized co-delivery system with lapatinib microparticles and paclitaxel nanoparticles in a peritumorally injectable in situ hydrogel. J. Control. Release 2015, 220, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals-special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Wu, L.B.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Res. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Park, J.; Sun, B.; Yeo, Y. Albumin-Coated Nanocrystals for Carrier-Free Delivery of Paclitaxel. J. Control. Release 2017, 263, 90–101. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Thompson, D.H.; Park, K.; Li, T. Impact of Surfactant Treatment of Paclitaxel Nanocrystals On Biodistribution and Tumor Accumulation in Tumor-Bearing Mice. J. Control. Release 2016, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.G.; Lv, F.M.; Wang, J.; Cao, S.J.; Liu, Z.P.; Liu, Y.; Lu, W.Y. RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability. Int. J. Pharm. 2019, 556, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the Adhesive Pads of Mytilus Edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H. Adhesion a La Moule. Integr. Comp. Biol. 2002, 42, 1172–1180. [Google Scholar] [CrossRef]
- Zhu, Y.; Chian, K.S.; Chan-Park, M.B.; Mhaisalkar, P.S.; Ratner, B.D. Protein Bonding On Biodegradable Poly (L-Lactide-Co-Caprolactone) Membrane for Esophageal Tissue Engineering. Biomaterials 2006, 27, 68–78. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.; Zeng, H.; Xu, Z.K. Deposition and Adhesion of Polydopamine On the Surfaces of Varying Wettability. ACS Appl. Mater. Interfaces 2017, 9, 30943–30950. [Google Scholar] [CrossRef]
- Gao, W.; Lee, D.; Meng, Z.J.; Li, T.L. Exploring intracellular fate of drug nanocrystals with crystal-integrated and environment-sensitive fluorophores. J. Control. Release 2017, 267, 214–222. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.L.; Ran, D.N.; Lu, L.W.; Zhan, C.Y.; Ruan, H.T.; Hu, X.F.; Xie, C.; Jiang, K.; Li, J.Y.; Zhou, J.F.; et al. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS Nano 2019, 13, 5591–5601. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, W.; Zhang, X.; Dong, Y.; Hua, Y.; Zhang, H.; Gao, J.; Zhao, L.; Li, Y.; Zheng, A. In Vitro and in Vivo Evaluation of Sn-38 Nanocrystals with Different Particle Sizes. Int. J. Nanomed. 2017, 12, 5487–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the TPE-labeled cNC@PDA-PEG and Mal-PEG3000-NH2 are not available from the authors. |










| Treatment (P:L) | IC50 of PTX (nM) | IC50 of LAPA (nM) | CI50 |
|---|---|---|---|
| PTX | 6965.00 | ||
| 10:1 | 1427.00 | 314.5 | 0.23 |
| 2:1 | 733.80 | 399.41 | 0.14 |
| 1:1 | 860.40 | 1264.00 | 0.22 |
| 1:2.5 | 807.8 | 2954.00 | 0.35 |
| 1:5 | 1151.00 | 8455.00 | 0.84 |
| 1:10 | 2141.00 | 22090.00 | 2.07 |
| LAPA | 12510.00 |
| Formulation | DL (PTX) | EE (PTX) | DL (LAPA) | EE (LAPA) |
|---|---|---|---|---|
| cNC | 32.35 ± 1.48 | 45.29 ± 2.42 | 15.31 ± 1.57 | 22.65 ± 3.14 |
| cNC@PDA | 25.38 ± 1.56 | 48.22 ± 1.96 | 13.33 ± 1.43 | 24.11 ± 1.86 |
| cNC@PDA-PEG | 21.33 ± 1.43 | 54.60 ± 1.39 | 10.95 ± 1.24 | 27.30 ± 2.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lv, F.-M.; Wang, D.-L.; Du, J.-L.; Guo, H.-Y.; Chen, H.-N.; Zhao, S.-J.; Liu, Z.-P.; Liu, Y. Synergistic Antitumor Effects on Drug-Resistant Breast Cancer of Paclitaxel/Lapatinib Composite Nanocrystals. Molecules 2020, 25, 604. https://doi.org/10.3390/molecules25030604
Wang J, Lv F-M, Wang D-L, Du J-L, Guo H-Y, Chen H-N, Zhao S-J, Liu Z-P, Liu Y. Synergistic Antitumor Effects on Drug-Resistant Breast Cancer of Paclitaxel/Lapatinib Composite Nanocrystals. Molecules. 2020; 25(3):604. https://doi.org/10.3390/molecules25030604
Chicago/Turabian StyleWang, Jun, Feng-Mei Lv, Dong-Li Wang, Jian-Liang Du, Hai-Yan Guo, Hai-Ni Chen, Shou-Jin Zhao, Zhe-Peng Liu, and Yu Liu. 2020. "Synergistic Antitumor Effects on Drug-Resistant Breast Cancer of Paclitaxel/Lapatinib Composite Nanocrystals" Molecules 25, no. 3: 604. https://doi.org/10.3390/molecules25030604