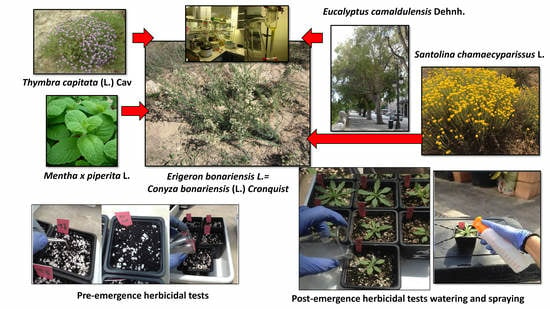
Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pre-Emergence Treatments
2.2. Post-Emergence Treatments
2.3. Essential Oils Composition
3. Materials and Methods
3.1. Erigeron Bonariensis L. Seeds
3.2. Essential Oils (EOs)
3.3. Gas Chromatography (GC)
3.4. Gas Chromatography−Mass Spectrometry (GC–MS)
3.5. Greenhouse Conditions
3.6. Pot Preparation
3.7. Pre-Emergence Herbicidal Tests
3.8. Post-Emergence Herbicidal Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP/248; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Hüter, O.F. Use of natural products in the crop protection industry. Phytochem. Rev. 2011, 10, 185–194. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Troyer, J.R. In the beginning: The multiple discovery of the first hormone herbicides. Weed Sci. 2001, 49, 290–297. [Google Scholar] [CrossRef]
- Catalá, R.; Salinas, J. Tailoring crop nutrition to fight weeds. Proc. Natl. Acad. Sci. USA 2018, 115, 7456–7458. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Limitations of existing weed control practices necessitate development of alternative techniques based on biological approaches. Adv. Agron. 2018, 147, 239–280. [Google Scholar]
- World Health Organization and Food; Agriculture Organization of the United Nations. The International Code of Conduct on Pesticide Management; Rome, Italy, 2014. Available online: http://www.fao.org/3/a-i3604e.pdf (accessed on 23 November 2019).
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the Development of Novel Strategies in Plant Disease Management. Front. Microbiol. 2017, 8, 654–664. [Google Scholar] [CrossRef] [Green Version]
- Manker, D.C. Natural products as green pesticides. In New Discoveries in Agrochemicals; Clark, J.M., Ohkawa, H., Eds.; American Chemical Society: Washington, DC, USA, 2005; pp. 283–294. [Google Scholar]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural hericides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blazquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Setia, N.; Kohli, R.K. Herbicidal activity of volatile oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 2005, 146, 89–94. [Google Scholar] [CrossRef]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean lamiaceae as weed germination inhibitors. J. Agric. Food. Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef] [PubMed]
- Frabboni, L.; Tarantino, A.; Petruzzi, F.; Disciglio, G. Bio-Herbicidal Effects of Oregano and Rosemary Essential Oils on Chamomile (Matricaria chamomilla L.) Crop in Organic Farming System. Agronomy (Basel) 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, A.A.; Sadia, S.; Ali, H.H.; Jabran, K.; Peerzada, A.M.; Chauhan, B.S. Biology and management of two important Conyza weeds: A global review. Environ. Sci. Poll. Res. 2016, 23, 24694–24710. [Google Scholar] [CrossRef]
- Michael, P.W. Some weedy species of Amaranthus (amaranths) and Conyza/Erigeron (fleabanes) naturalised in the Asian-Pacific region. In Proceedings of the 6th Asian-Pacific Weed Science Society Conference, Jakarta, Indonesia, 11–17 July 1977; Asian-Pacific Weed Science Society: Jakarta, Indonesia, 1977; pp. 87–95. [Google Scholar]
- Trezzi, M.M.; Balbinot, A.A., Jr.; Benin, G.; Debastiani, F.; Patel, F.; Miotto, E., Jr. Competitive ability of soybean cultivars with horseweed (Conyza bonariensis). Planta Daninha 2013, 31, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.A.; Galon, L.; Aspiazu, I.; Silva, A.A.; Concenco, G.; Silva, A.F.; Oliveira, J.A.; Vargas, L. Glyphosate translocation in hairy fleabane (Conyza bonariensis) biotypes. Planta Daninha 2008, 26, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Walker, S.; Rollin, M.J.; Tan, D.K.Y.; Robinson, G.; Werth, J. Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biol. Manag. 2007, 7, 192–199. [Google Scholar] [CrossRef]
- Widderick, M.; Walker, S.; Cook, T. Flaxleaf fleabane (Conyza bonariensis)-strategic solutions using best management practice. Pak. J. Weed Sci. Res. 2012, 18, 687–693. [Google Scholar]
- Bhowmik, P.C.; Bekech, M.M. Horseweed (Conyza canadensis) seed production, emergence and distribution in no-tillage and conventional-tillage corn (Zea mays). Agron. Trends Agric. Sci. 1993, 11, 67–71. [Google Scholar]
- Wu, H.; Walker, S.; Robinson, G.; Coombes, N. Control of flaxleaf fleabane (Conyza bonariensis) in wheat and sorghum. Weed Technol. 2010, 24, 102–107. [Google Scholar] [CrossRef]
- Moreira, M.S.; Nicolai, M.; Carvalho, S.J.P.; Christoffoleti, P.J. Resistência de Conyza canadensis e C. bonariensis ao herbicida glyphosate. Planta Daninha 2007, 25, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org. (accessed on 16 November 2019).
- Garcia Plasencia, S. Actividad herbicida del aceite esencial de Thymus capitatus (L.) Hoffmanns. et Link. y su efectividad en función de distintos métodos de aplicación. Master’s Thesis, Universitat Politècnica de València, Valencia, Spain, 24 September 2013. [Google Scholar]
- Hanana, M.; Mansour, M.B.; Algabr, M.; Amri, I.; Gargouri, S.; Romane, A.; Jamoussi, B.; Hamrouni, L. Potential use of essential oils from four Tunisian species of Lamiaceae: Biological alternative for fungal and weed control. Rec. Nat. Prod. 2017, 11, 258–269. [Google Scholar]
- Verdeguer, M. Fitotoxicidad de Aceites Esenciales y Extractos Acuosos de Plantas Mediterráneas para el Control de Arvenses. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 14 October 2011. [Google Scholar]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha×piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Miceli, A.; Negro, C.; Tommasi, L. Essential oil variability in Thymbra capitata (L.) Cav. growing wild in Southern Apulia (Italy). Biochem. Syst. Ecol. 2006, 34, 528–535. [Google Scholar] [CrossRef]
- Fleisher, Z.; Fleisher, A. Volatiles of Coridothymus capitatus Chemotypes Growing in Israel: Aromatic Plants of the Holy Land and the Sinai. Part XV J. Essent. Oil Res. 2002, 14, 105–106. [Google Scholar] [CrossRef]
- Hedhili, L.; Romdhane, M.; Abderrabba, M.; Planche, H.; Cherif, I. Variability in essential oil composition of Tunisian Thymus capitatus (L.) Hoff. et Link. Flavour Frag. J. 2002, 17, 26–28. [Google Scholar] [CrossRef]
- Saoud, I.; Hamrouni, L.; Gargouri, S.; Amri, I.; Hanana, M.; Fezzani, T. Chemical composition, weed killer and antifungal activities of Tunisian thyme (Thymus capitatus Hoff. et Link.) essential oils. Acta Aliment. Hung. 2013, 42, 417–427. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017, 28, 1168–1180. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, P.F.; Costa, A.V.; Alves, T.D.A. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 2015, 63, 8981–8990. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Paschalidis, K.; Christos, R. Herbicidal potential on Lolium rigidum of nineteen major essential oil components and their synergy. J. Essent. Oil Res. 2013, 25, 1–10. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.G.; Blionis, J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.D.; Mancini, E.; de Almeida, L.F.R.; Feo, V.D. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaimovitsh, D.; Shachter, A.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2017, 65, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical composition and biological activities of Mentha species. In Aromatic and Medicinal Plants—Back to Nature; InTech: London, UK, 2017; pp. 47–80. [Google Scholar]
- Soković, M.D.; Glamočlija, J.; Marin, P.D.; Brkić, D.D.; Vukojević, J.; Jovanović, D.; Bulajić, N.; Kataranovski, D. Antifungal Activity of the Essential Oil of Mentha × piperita. Pharm. Biol. 2006, 44, 511–515. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × Piperita, L. (peppermint) essential oils. J. King Saud Univ. Sci. 2017, 31, 528–533. [Google Scholar] [CrossRef]
- Synowiec, A.; Drozdek, E. Physicochemical and herbicidal properties of emulsions of essential oils against Avena fatua L. and Chenopodium album L. J. Plant. Dis. Prot. 2016, 123, 65–74. [Google Scholar] [CrossRef]
- Maffei, M.; Wanda, C.; Silvano, S. Effect of Mentha × piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 2001, 58, 703–707. [Google Scholar] [CrossRef]
- Skrzypek, E.; Repka, P.; Stachurska-Swakon, A.; Barabasz-Krasny, B.; Mozdzen, K. Allelopathic effect of aqueous extracts from the leaves of peppermint (Mentha × piperita L.) on selected physiological processes of common sunflower (Helianthus annuus L.). Not. Bot Horti. Agrobo. 2015, 43, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Campiglia, E.; Mancinelli, R.; Cavalieri, A.; Caporali, F. Use of essential oils of cinnamon (Cinnamomum zeylanicum L.), lavender (Lavandula spp.) and peppermint (Mentha × piperita L.) for weed control. Ital. J. Agron. 2007, 58, 171–175. [Google Scholar] [CrossRef]
- Pappas, R.S.; Sheppard-Hanger, S. Essential oil of Eucalyptus camaldulensis Dehn. from south Florida: A high cryptone/low cineole eucalyptus. J. Essent. Oil Res. 2000, 12, 383–384. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Kundakovic, T.; Gorunovic, M.S. Essential oil from leaves of Eucalyptus camaldulensis Dehn, Myrtaceae from Jerusalem. J. Essent. Oil Res. 2001, 13, 105–107. [Google Scholar] [CrossRef]
- Tsiri, D.; Kretsi, O.; Chinou, I.B.; Spyropoulos, C.G. Composition of fruit volatiles and annual changes in the volatiles of leaves of Eucalyptus camaldulensis Dehn. Growing in Greece. Flavour Fragr. J. 2003, 18, 244–247. [Google Scholar] [CrossRef]
- Üstüner, T.; Kordali, S.; Bozhüyük, A.U. Investigation of pesticidal activities of essential oil of Eucalyptus camaldulensis Dehnh. Rec. Nat. Prod. 2018, 12, 557–568. [Google Scholar] [CrossRef]
- Fouad, R.; Bousta, D.; Lalami, A.E.; Chahdi, F.O.; Amri, I.; Jamoussi, B.; Greche, H. Chemical composition and herbicidal effects of essential oils of Cymbopogon citratus (DC) Stapf, Eucalyptus cladocalyx, Origanum vulgare L and Artemisia absinthium L cultivated in Morocco. J. Essent. Oil Bear. Plants 2015, 18, 112–123. [Google Scholar] [CrossRef]
- Vernin, G. Volatile constituents of the essential oil of Santolina chamaecyparissus L. J. Essent. Oil Res. 1991, 3, 49–53. [Google Scholar] [CrossRef]
- Perez-Alonso, M.J.; Velasco-Negueruela, A. Essential oil components of Santolina chamaecyparissus. Flavour Fragrance J. 1992, 7, 37–41. [Google Scholar] [CrossRef]
- Derbesy, M.; Touche, J.; Zola, A. The essential oil of Santolina chamaecyparissus L. J. Ess. Oil Res. 1989, 1, 269–275. [Google Scholar] [CrossRef]
- Grosso, C.; Coelho, J.A.; Urieta, J.S.; Palavra, A.M.F.; Barroso, J.G. Herbicidal activity of volatiles from coriander, winter savory, cotton lavender, and thyme isolated by hydrodistillation and supercritical fluid extraction. J. Agric. Food Chem. 2010, 58, 11007–11013. [Google Scholar] [CrossRef]
- Garg, S.N.; Gupta, D.; Mehta, V.K.; Kumar, S. Volatile Constituents of the Essential Oil of Santolina chamaecyparissus Linn, from the Southern Hills of India. J. Essent. Oil Res. 2001, 13, 234–235. [Google Scholar] [CrossRef]
- De Elguea-Culebras, G.O.; Sanchez-Vioque, R.; Berruga, M.I.; Herraiz-Penalver, D.; Gonzalez-Coloma, A.; Andres, M.F.; Santana-Meridas, O. Biocidal potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula × intermedia var. super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Carretero, J.L. Flora Arvense Española: Las Malas Hierbas de los Cultivos Españoles; Phytoma: Valencia, España, 2004. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ranal, M.A.; Garcia De Santana, D. How and why to measure the germination process? Revista Brasil. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ranal, M.A.; Garcia De Santana, D.; Resende Ferreira, W.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Revista Brasil. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
Sample Availability: Samples of the essential oils tested are available from the authors. |






| G (%) | MT (days) | CVt (%) | MR (days−1) | U (bit) | Z | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | Mean ± st.dev. | DF | Mean ± st.dev. | DF | Mean ± st.dev. | DF | Mean ± st.dev. | DF | Mean ± st.dev. | DF | Mean ± st.dev. | ||||||||
| Treatment (T) | 5 | 5 | 5 | 5 | 5 | 4 | |||||||||||||
| Water (C1) | 28.00 ± 21.50 | B | 13.23 ± 9.07 | C | 42.10 ± 35.75 | B | 0.1033 ± 0.0527 | AB | 0.28 ± 0.46 | AB | 0.00 ± 0.00 | ||||||||
| Water + Fitoil (C2) | 54.00 ± 18.97 | A | 9.96 ± 2.82 | B | 135.50 ± 16.24 | A | 0.1097 ± 0.0387 | A | 0.56 ± 0.49 | A | 0.50 ± 0.41 | ||||||||
| E. camaldulensis | 8.00 ± 13.49 | CD | 27.78 ± 12.28 | A | 149.73 ± 13.17 | A | 0.0474 ± 0.0309 | B | 0.07 ± 0.25 | B | 0.33 ± 0.58 | ||||||||
| M. piperita | 14.67 ± 16.55 | BC | 19.32 ± 10.14 | A | 139.95 ± 5.01 | A | 0.0662 ± 0.0307 | AB | 0.09 ± 0.34 | B | 0.60 ± 0.55 | ||||||||
| S. chamaecyparissus | 9.33 ± 15.52 | CD | 21.67 ± 12.62 | A | 142.24 ± 1.42 | A | 0.0767 ± 0.0713 | AB | 0.05 ± 0.29 | B | 0.67 ± 0.58 | ||||||||
| T. capitata | 0.67 ± 3.65 | D | 30.00 ± 00.00 | A | 0.0333 ± 0.0000 | AB | 0.00 ± 0.00 | B | |||||||||||
| F(5,126) = 27.82 *** | F(5,43) = 8.46 *** | F(5,18) = 22.62 *** | F(5,42) = 3.53 ** | F(5,126) = 6.80 *** | F(4,18) = 1.49 n.s. | ||||||||||||||
| Dose (D) within treatment (T) | 8 | 5 | 1 | 5 | 8 | 1 | |||||||||||||
| E. camaldulensis | 2 µL | 18.00 ± 17.51 | 23.33 ± 12.52 | 149.73 a ± 13.17 | 0.0573 ± 0.0343 | 0.20 ± 0.42 | 0.33 ± 0.58 | ||||||||||||
| 4 µL | 6.00 a ± 9.66 | 36.67 a ± 5.77 | 0.0278 a ± 0.0048 | 0.00 ± 0.00 | |||||||||||||||
| 8 µL | 0.00 a ± 0.00 | 0.00 ± 0.00 | |||||||||||||||||
| M. piperita | 2 µL | 32.00 ± 13.98 | 12.92 ± 5.26 | 139.95 ± 5.01 | 0.0851 ± 0.0219 | 0.26 ± 0.56 | 0.60 ± 0.55 | ||||||||||||
| 4 µL | 10.00 a ± 10.54 | 28.00 a ± 4.47 | 0.0367 a ± 0.0075 | 0.00 ± 0.00 | |||||||||||||||
| 8 µL | 2.00 a ± 6.32 | 40.00 a ± 0.00 | 0.0250 ± 0.0000 | 0.00 ± 0.00 | |||||||||||||||
| S. chamaecyparissus | 2 µL | 18.00 ± 19.89 | 20.28 ± 12.54 | 142.65 a ± 1.73 | 0.0770 ± 0.0709 | 0.16 ± 0.50 | 0.50 ± 0.71 | ||||||||||||
| 4 µL | 6.00 a ± 9.66 | 18.33 ± 11.55 | 0.0933 ± 0.0924 | 0.00 ± 0.00 | |||||||||||||||
| 8 µL | 4.00 a ± 12.65 | 40.00 ± 0.00 | 141.42 ± 0.00 | 0.0250 ± 0.0000 | 0.00 ± 0.00 | 1.00 ± 0.00 | |||||||||||||
| T. capitata | 2 µL | 2.00 a ± 6.32 | 30.00 ± 0.00 | 0.0333 ± 0.0000 | 0.00 a ± 0.00 | ||||||||||||||
| 4 µL | 0.00 a ± 0.00 | 0.00 a ± 0.00 | |||||||||||||||||
| 8 µL | 0.00 a ± 0.00 | 0.00 a ± 0.00 | |||||||||||||||||
| F(8,126) = 7.33 *** | F(5,43) = 5.83 *** | F(1,18) < 1 n.s. | F(5,42) = 1.55 n.s. | F(8,126) = 1.29 n.s. | F(1,18) < 1 n.s. | ||||||||||||||
| Error | 126 | 43 | 18 | 42 | 126 | 18 | |||||||||||||
| Total | 139 | 53 | 23 | 52 | 139 | 23 | |||||||||||||
| Phytotoxicity | |||
|---|---|---|---|
| Source | DF | Mean ± st. dev. | |
| Method of application (M) | 1 | ||
| Watering | 11.85 ± 47.38 | ||
| Spraying | 15.81 ± 46.41 | ||
| F (1, 2148) = 4.73 * | |||
| day (d) | 6 | ||
| 1 | 2.59 ± 21.07 | ||
| 5 | 4.33 ± 22.52 | ||
| 10 | 17.87 ± 51.99 | ||
| 15 | 20.08 ± 52.26 | ||
| 20 | 16.76 ± 53.92 | ||
| 25 | 16.56 ± 54.66 | ||
| 30 | 18.62 ± 53.54 | ||
| F (6, 2148) = 9.33 *** | |||
| Treatment (T) | 5 | ||
| Water (C1) | −16.28 ± 25.75 | ||
| Water + Fitoil (C2) | −18.66 ± 26.76 | ||
| E. camaldulensis | 17.42 ± 45.48 | ||
| M. piperita | 21.67 ± 42.36 | ||
| S. chamaecyparissus | 1.08 ± 33.84 | ||
| T. capitata | 56.9 ± 49.34 | ||
| F (5, 2148) = 271.91 *** | |||
| Dose within treatment (T) | 8 | F (8, 2148) = 61.2 *** | |
| M*d | 6 | F (6, 2148) < 1 n.s. | |
| M*T | 5 | F (5, 2148) = 48.87 *** | |
| d*T | 30 | F (30, 2148) = 9.19 *** | |
| M*d*T | 30 | F (30, 2148) = 2.38 *** | |
| Error | 2148 | ||
| Total | 2239 | ||
| Component | KI. | TC | MP | EC | SC |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 22.54 | 1.95 | 22.27 | 9.30 | |
| Santolina triene | 908 | - | - | - | 0.13 |
| α-Thujene | 930 | 0.89 | 0.01 | 0.43 | - |
| α-Pinene | 938 | 0.74 | 0.28 | - | 0.85 |
| Thuja-2,4(10)-diene | 947 | - | - | 0.10 | - |
| Camphene | 951 | - | - | - | 0.28 |
| Sabinene | 975 | - | 0.14 | 0.09 | 0.17 |
| β-Pinene | 978 | 0.29 | 0.43 | - | 3.98 |
| Myrcene | 991 | 1.95 | 0.01 | 0.08 | - |
| α-Phellandrene | 1004 | 0.16 | - | 0.11 | - |
| γ-Terpinene | 1016 | 7.77 | 0.13 | 0.12 | 1.18 |
| α-Terpinene | 1016 | 1.61 | - | - | 0.69 |
| p-Cymene | 1025 | 8.93 | 0.18 | 20.36 | 2.01 |
| Limonene | 1029 | 0.20 | 0.73 | 0.87 | - |
| (Z)-β-Ocimene | 1040 | - | 0.03 | - | - |
| iso-Terpinolene | 1087 | - | 0.02 | - | - |
| p-Cymenene | 1090 | - | - | 0.11 | - |
| Oxygenated monoterpens | 73.98 | 95.35 | 33.76 | 39.32 | |
| 1,8-Cineole | 1031 | 0.11 | 4.31 | 2.31 | 17.50 |
| trans-Pinocarveol | 1037 | - | - | - | 0.17 |
| Artemisia ketone | 1062 | - | - | - | 4.63 |
| (Z)-Sabinene hydrate | 1070 | - | 0.76 | - | - |
| Linalool | 1097 | 0.77 | 0.09 | - | 0.42 |
| trans-Thujone | 1117 | - | - | 0.19 | - |
| Camphor | 1142 | - | - | - | 4.03 |
| Menthone | 1154 | - | 20.52 | - | - |
| (E)-Pinocamphone | 1159 | - | - | - | 0.18 |
| (Z)-Chrysanthemol | 1162 | - | - | - | 3.80 |
| Menthofuran | 1163 | - | 5.21 | - | - |
| neo-Menthol | 1165 | - | 3.12 | - | - |
| Borneol | 1168 | 0.16 | - | - | 1.11 |
| (Z)-Pinocamphone | 1172 | - | - | - | 2.03 |
| Menthol | 1175 | - | 51.81 | - | - |
| Terpinen-4-ol | 1177 | 0.37 | 0.67 | 2.89 | 2.69 |
| iso-menthol | 1182 | - | 0.60 | - | - |
| Neoisomenthol | 1187 | - | 0.08 | - | - |
| α-Terpineol | 1188 | - | 0.17 | 0.93 | 0.21 |
| Myrtenal | 1192 | - | - | - | 1.31 |
| Myrtenol | 1193 | - | - | - | 1.07 |
| Cryptone | 1196 | - | - | 17.00 | - |
| Verbenone | 1198 | - | - | - | 0.16 |
| m-Cumenol | 1230 | - | - | 0.46 | - |
| Pulegone | 1236 | - | 0.83 | - | - |
| Cumin aldehyde | 1245 | - | 4.15 | - | |
| Carvotanacetone | 1250 | - | - | 0.33 | - |
| Piperitone | 1251 | - | 0.32 | - | - |
| neo-Menthyl acetate | 1273 | - | 0.16 | - | - |
| p-Menth-1-en-7-al | 1279 | - | - | 2.85 | - |
| Menthyl acetate | 1291 | - | 6.56 | - | - |
| Thymol | 1292 | 0.27 | - | 1.14 | - |
| Carvacrol | 1300 | 72.30 | - | 1.51 | - |
| iso-Menthyl acetate | 1303 | - | 0.16 | - | - |
| Sesquiterpene hydrocarbons | 3.14 | 2.22 | 0.19 | 21.78 | |
| α-Ylangene | 1373 | - | - | - | 0.08 |
| α-Bourbonene | 1381 | - | 0.17 | - | - |
| β-Caryophyllene | 1415 | 3.14 | 1.47 | - | 0.39 |
| β-Farnesene | 1454 | - | 0.02 | - | - |
| allo-Aromadendrene | 1457 | - | - | 0.19 | 4.23 |
| trans-Cadina-1(6),4-diene | 1473 | 0.36 | |||
| Germacrene-D | 1477 | - | 0.42 | - | 12.60 |
| β-Selinene | 1491 | - | 0.13 | - | - |
| Elixene | 1492 | - | - | - | 2.80 |
| γ-Cadinene | 1509 | - | - | - | 0.32 |
| δ-Cadinene | 1519 | - | - | - | 1.00 |
| Oxygenated sesquiterpenes | 0.14 | 0.00 | 34.47 | 15.64 | |
| Bornyl acetate | 1283 | - | - | - | 0.08 |
| Spathulenol | 1477 | - | - | 31.29 | 1.42 |
| Caryophyllene oxide | 1577 | 0.14 | - | - | 0.19 |
| Viridiflorol | 1587 | - | - | 0.54 | 13.56 |
| β-Oplopenone | 1602 | - | - | - | 0.16 |
| Spathulenol isomer | 1616 | - | - | 1.29 | - |
| iso-spathulenol | 1640 | - | - | 1.35 | - |
| α-Cadinol | 1649 | - | - | - | 0.23 |
| Others | 0.00 | 0.14 | 0.00 | 12.91 | |
| 1-Butanol, 2-methyl-, propanoate | 973 | - | - | - | 0.20 |
| 1-Octen-3-ol | 980 | - | 0.02 | - | - |
| 3-Octanol | 995 | - | 0.07 | - | - |
| iso-Amyl 2-methyl butyrate | 1101 | - | 0.02 | - | - |
| n-Amyl isovalerate | 1106 | - | 0.04 | - | 0.48 |
| 8-methylene-3-oxatricyclo[5,2,0,0(2,4)]nonane | 1117 | - | - | - | 12.24 |
| TOTAL IDENTIFIED (%) | 99.80 | 99.66 | 90.69 | 98.95 |
| Month | Temperature (°C) | Relative Humidity (R.H.) % | ||||
|---|---|---|---|---|---|---|
| Mean | Maximum | Minimum | Mean | Maximum | Minimum | |
| May | 25.0 | 33.2 | 19.2 | 64.5 | 81.2 | 32.3 |
| June | 27.9 | 36.3 | 20.1 | 61.4 | 82.1 | 24.8 |
| July | 27.9 | 35.6 | 19.1 | 69.0 | 93.4 | 36.8 |
| August | 28.4 | 36.5 | 22.3 | 68.4 | 86.7 | 42.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils. Molecules 2020, 25, 562. https://doi.org/10.3390/molecules25030562
Verdeguer M, Castañeda LG, Torres-Pagan N, Llorens-Molina JA, Carrubba A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils. Molecules. 2020; 25(3):562. https://doi.org/10.3390/molecules25030562
Chicago/Turabian StyleVerdeguer, Mercedes, Luis Guillermo Castañeda, Natalia Torres-Pagan, Juan Antonio Llorens-Molina, and Alessandra Carrubba. 2020. "Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils" Molecules 25, no. 3: 562. https://doi.org/10.3390/molecules25030562