Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas
Abstract
:1. Introduction
2. Results
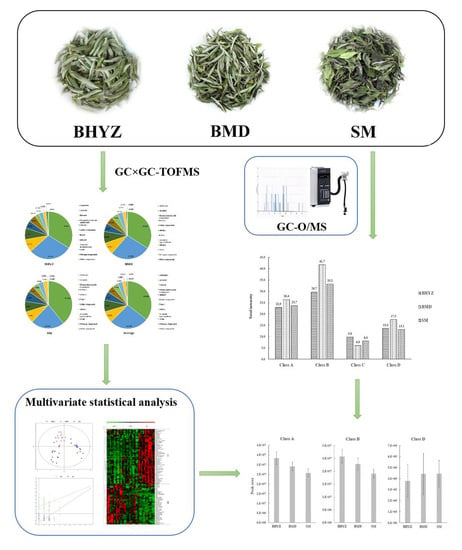
2.1. The Aroma Profile in Different Subtypes of White Teas
2.2. Screening of Differential Volatile Compounds among the Three Subtypes of White Teas
2.3. Identification of Aroma-Active Compounds in White Teas Using GC–O/MS
3. Discussion
3.1. The Comparison of Obtained and Reported Aroma Profiles of White Teas
3.2. The Comparison of Obtained and Reported Aroma-Active Compounds in Teas
3.3. The Comparison of Total Aroma Intensities of Aroma-Active Compounds among Different Subtypes of White Teas
3.4. The Comparison of the Results Obtained from GC × GC–TOFMS and GC–O/MS
3.5. Determination of Key Odorants Accounting for Aroma Characteristics of the Three Subtypes of White Teas
4. Materials and Methods
4.1. Tea Samples
4.2. Chemicals and Reagents
4.3. Headspace Solid-Phase Microextraction (HS-SPME)
4.4. GC × GC–TOFMS Analysis
4.5. GC–O/MS Analysis
4.6. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanlier, N.; Atik, I.; Atik, A. A minireview of effects of white tea consumption on diseases. Trends Food Sci. Tech. 2018, 82, 82–88. [Google Scholar]
- Yang, C.; Hu, Z.; Lu, M.; Li, P.; Tan, J.; Chen, M.; Lv, H.; Zhu, Y.; Zhang, Y.; Guo, L.; et al. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int. 2018, 106, 909–919. [Google Scholar] [PubMed]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [PubMed]
- Tan, J.; Engelhardt, U.H.; Lin, Z.; Kaiser, N.; Maiwald, B. Flavonoids, phenolic acids, alkaloids and theanine in different types of authentic Chinese white tea samples. J. Food Compos. Anal. 2017, 57, 8–15. [Google Scholar]
- Zhao, F.; Qiu, X.; Ye, N.; Qian, J.; Wang, D.; Zhou, P.; Chen, M. Hydrophilic interaction liquid chromatography coupled with quadrupole-orbitrap ultra high resolution mass spectrometry to quantitate nucleobases, nucleosides, and nucleotides during white tea withering process. Food Chem. 2018, 266, 343–349. [Google Scholar]
- Yue, W.; Sun, W.; Rao, R.S.P.; Ye, N.; Yang, Z.; Chen, M. Non-targeted metabolomics reveals distinct chemical compositions among different grades of Bai Mudan white tea. Food Chem. 2019, 277, 289–297. [Google Scholar]
- Wang, L.; Cai, L.S.; Lin, Z.; Zhong, Q.S.; Lv, H.P.; Tan, J.F.; Guo, L. Analysis of aroma compounds in white tea using headspace solid-phase micro-extraction and GC-MS. J. Tea Sci. 2010, 30, 115–123. (In Chinese) [Google Scholar]
- Wang, K.; Liu, F.; Liu, Z.; Huang, J.; Xu, Z.; Li, Y.; Chen, J.; Gong, Y.; Yang, X. Comparison of catechins and volatile compounds among different types of tea using high performance liquid chromatograph and gas chromatograph mass spectrometer. Int. J. Food Sci. Tech. 2011, 46, 1406–1412. [Google Scholar]
- Zhu, Y.; Shao, C.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; Lin, Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis). J. Chromatogr. A 2017, 1490, 177–190. [Google Scholar]
- Qi, D.; Miao, A.; Cao, J.; Wang, W.; Chen, W.; Pang, S.; He, X.; Ma, C. Study on the effects of rapid aging technology on the aroma quality of white tea using GC-MS combined with chemometrics: In comparison with natural aged and fresh white tea. Food Chem. 2018, 265, 189–199. [Google Scholar]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.P.; Dai, W.D.; Guo, L.; Tan, J.F.; Zhang, Y.; Yu, F.L.; Shao, C.Y.; Peng, Q.H.; Lin, Z. Separation of aroma components in Xihu Longjing tea using simultaneous distillation extraction with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Sep. Purif. Technol. 2016, 164, 146–154. [Google Scholar] [CrossRef]
- Lv, H.; Zhong, Q.; Lin, Z.; Wang, L.; Tan, J.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC-olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lu, X.; Xu, G. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and multivariate data analysis. J. Chromatogr. A 2013, 1313, 245–252. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Human Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef] [Green Version]
- Plutowska, B.; Wardencki, W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Baba, R.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of Chinese green tea infusions by aroma extract dilution analysis. J. Agric. Food Chem. 2014, 62, 8308–8313. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black yea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Li, C.; Liu, S.; Zhang, C.; Li, L.; Jiang, D. Characterization of aroma-active compounds of Pu-erh tea by headspace solid-phase microextraction (HS-SPME) and simultaneous distillation-extraction (SDE) coupled with GC-olfactometry and GC-MS. Food Anal. Method 2016, 9, 1118–1198. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, C.; Wang, W.; Hu, H.; Chen, H.; Pang, S.; Miao, A. Effects of withering duration on the aroma profile of Yinghong No. 9 white tea. Food Sci. 2017, 38, 138–143. (In Chinese) [Google Scholar]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of matcha by gas chromatography-olfactometry techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef]
- Jumtee, K.; Komura, H.; Bamba, T.; Fukusaki, E. Predication of Japanese green tea (Sen-cha) ranking by volatile profiling using gas chromatography mass spectrometry and multivariate analysis. J. Biosci. Bioeng. 2011, 112, 252–255. [Google Scholar] [CrossRef]
- Tadmor, Y.; Fridman, E.; Gur, A.; Larkov, O.; Lastochkin, E.; Ravid, U.; Zamir, D.; Lewinsohn, E. Identification of malodorous, a wild species allele affecting tomato aroma that was selected against during domestication. J. Agric. Food Chem. 2002, 50, 2005–2009. [Google Scholar] [CrossRef]






| No. | Compounds | BHYZ | BMD | SM | Average |
|---|---|---|---|---|---|
| 1 | Benzaldehyde ** | 160.4 ± 19.4 | 156.8 ± 17.7 | 128.0 ± 7.8 | 148.4 ± 20.9 |
| 2 | Linalool | 67.3 ± 4.6 | 71.7 ± 2.8 | 77.5 ± 9.8 | 72.2 ± 7.4 |
| 3 | Phenylethyl alcohol ** | 101.2 ± 22.9 | 68.9 ± 16.2 | 45.9 ± 5.0 | 72.0 ± 28.0 |
| 4 | (E)-2-Hexenal ** | 54.8 ± 9.3 | 54.8 ± 9.1 | 86.3 ± 12.6 | 65.3 ± 18.2 |
| 5 | Dimethyl sulfide ** | 49.5 ± 9.7 | 54.8 ± 12.5 | 37.8 ± 11.3 | 47.4 ± 12.7 |
| 6 | 2-Pentyl-furan | 42.3 ± 6.7 | 46.4 ± 12.6 | 52.3 ± 6.3 | 47.0 ± 9.3 |
| 7 | Geraniol ** | 47.7 ± 10.1 | 56.1 ± 15.2 | 26.8 ± 3.6 | 43.5 ± 16.2 |
| 8 | Decane | 29.2 ± 7.1 | 30.1 ± 2.4 | 38.3 ± 6.8 | 32.5 ± 6.9 |
| 9 | Methyl salicylate | 23.0 ± 6.9 | 37.3 ± 14.8 | 34.3 ± 13.8 | 31.5 ± 13.1 |
| 10 | Hexanal ** | 31.5 ± 3.6 | 25.4 ± 4.7 | 27.6 ± 7.6 | 28.1 ± 5.8 |
| 11 | Benzyl alcohol ** | 32.4 ± 1.1 | 26.7 ± 4.8 | 18.1 ± 3.0 | 25.7 ± 6.8 |
| 12 | 2-Ethyl-furan ** | 25.1 ± 5.0 | 24.1 ± 10.6 | 16.9 ± 3.3 | 22.0 ± 7.5 |
| 13 | Benzene acetaldehyde ** | 8.9 ± 2.1 | 17.5 ± 4.5 | 33.3 ± 14.2 | 19.9 ± 13.2 |
| 14 | 2-Methyl-butanal | 14.1 ± 3.3 | 14.0 ± 3.7 | 20.4 ± 9.3 | 16.1 ± 6.4 |
| 15 | (Z)-3-Hexen-1-ol | 13.1 ± 3.0 | 13.5 ± 2.0 | 16.8 ± 5.2 | 14.5 ± 3.8 |
| 16 | 3,5-Octadien-2-one isomer 1 * | 15.3 ± 5.0 | 15.4 ± 8.2 | 10.7 ± 3.1 | 13.8 ± 5.8 |
| 17 | Nonanal ** | 16.8 ± 4.2 | 13.3 ± 4.7 | 6.8 ± 1.6 | 12.3 ± 5.5 |
| 18 | 3,5-Octadien-2-one isomer 2 ** | 9.2 ± 2.2 | 9.3 ± 4.3 | 19.4 ± 5.9 | 12.6 ± 6.4 |
| 19 | 6-Methyl-5-hepten-2-one * | 10.4 ± 1.1 | 8.5 ± 1.7 | 10.3 ± 1.4 | 9.7 ± 1.6 |
| 20 | 2-Heptanone ** | 11.0 ± 2.1 | 9.4 ± 1.9 | 7.8 ± 1.5 | 9.4 ± 2.2 |
| 21 | Butanal ** | 12.5 ± 1.8 | 9.8 ± 1.9 | 5.9 ± 2.7 | 9.4 ± 3.5 |
| 22 | 2,4-Heptadienal isomer 2 ** | 4.7 ± 0.8 | 6.1 ± 2.8 | 15.7 ± 9.2 | 8.8 ± 7.2 |
| 23 | β-Myrcene ** | 8.6 ± 1.7 | 9.4 ± 0.9 | 7.5 ± 1.1 | 8.5 ± 1.4 |
| 24 | Toluene ** | 6.4 ± 1.5 | 7.3 ± 1.6 | 9.5 ± 1.4 | 7.8 ± 1.9 |
| 25 | Dodecane | 6.9 ± 0.8 | 7.1 ± 1.5 | 8.6 ± 1.8 | 7.5 ± 1.5 |
| 26 | Hexanoic acid ** | 11.1 ± 6.0 | 6.8 ± 1.8 | 2.8 ± 0.5 | 6.9 ± 4.9 |
| 27 | 1-Octen-3-ol | 7.3 ± 0.7 | 6.1 ± 1.5 | 7.1 ± 1.5 | 6.8 ± 1.3 |
| 28 | 1-Penten-3-ol ** | 6.6 ± 1.4 | 6.1 ± 0.9 | 4.1 ± 0.7 | 5.6 ± 1.5 |
| 29 | 5-Ethyl-6-methyl-3-hepten-2-one | 5.7 ± 1.4 | 6.0 ± 0.4 | 5.0 ± 1.2 | 5.6 ± 1.1 |
| 30 | Limonene | 3.8 ± 0.9 | 5.4 ± 2.2 | 5.0 ± 0.6 | 4.8 ± 1.5 |
| No. | Compounds | Odor Characteristic | Aroma Intensity [1] | Identification Method [2] | Class [3] | ||
|---|---|---|---|---|---|---|---|
| BHYZ | BMD | SM | |||||
| 1 | Hexanal | Green, grassy, fresh | 2.0 | 2.8 | 2.6 | MS, RI | A |
| 2 | 1-Octen-3-ol | Mushroom-like, fresh | 2.0 | 2.5 | 3.0 | MS, RI, STD | A |
| 3 | β-Cymene | Floral, fruity, rice-like | 1.3 | MS, RI | B | ||
| 4 | Benzyl alcohol | Floral, rose-like, pollen-like | 2.0 | 3.0 | 2.3 | MS, RI | B |
| 5 | Benzene acetaldehyde | Green, sweet, green lemon-like | 3.0 | 3.2 | 2.8 | MS, RI, STD | B |
| 6 | (E)-2-Octenal | Stinky, green, sauce-like | 2.0 | 1.7 | MS, RI, STD | A | |
| 7 | trans-Linalool oxide (furanoid) | Floral, sweet, roasted | 1.7 | 2.0 | 2.7 | MS, RI, STD | B |
| 8 | 3,5-Octadien-2-one isomer 2 | Green, fresh, sweet | 2.5 | 3.2 | 2.8 | MS, RI, STD | A |
| 9 | Linalool | Floral, fruity, sweet | 2.8 | 3.4 | 3.2 | MS, RI, STD | B |
| 10 | Nonanal | Floral, fruity, green, fresh | 3.0 | 3.6 | 3.5 | MS, RI, STD | B |
| 11 | Phenylethyl alcohol | Floral, sweet, rose-like | 2.6 | 2.5 | 2.0 | MS, RI | B |
| 12 | 5-Ethyl-6-methyl-3-hepten-2-one | Stinky, grassy, fresh | 2.5 | 3.0 | 2.8 | MS, RI | A |
| 13 | (E)-3-Nonen-1-ol | Green, grassy, stinky | 3.0 | 3.2 | 3.6 | MS, RI, STD | A |
| 14 | (E)-2-Nonenal | Stinky, fresh, cucumber-like | 3.0 | 2.5 | MS, RI, STD | A | |
| 15 | 1-Nonanol | Green, fresh, grassy | 2.0 | 2.0 | MS, RI, STD | A | |
| 16 | Naphthalene | Non-pleasant, irritating | 2.0 | 2.3 | 3.3 | MS, RI, STD | D |
| 17 | cis-3-Hexenyl butyrate | Fresh, cucumber-like | 2.3 | MS, RI, STD | A | ||
| 18 | Terpineol | Floral, sweet, green, fresh | 2.0 | 2.7 | 2.0 | MS, RI | B |
| 19 | Neral | Floral, fruity, hay-like | 1.7 | MS, RI | B | ||
| 20 | cis-3-Hexenyl isovalerate | Green, fresh, sweet, floral | 2.2 | 3.0 | 2.6 | MS, RI, STD | A |
| 21 | Geraniol | Floral, rose-like, fresh | 2.2 | 2.6 | 3.2 | MS, RI | B |
| 22 | γ-Nonalactone | Sweet, cream-like, floral | 2.0 | 2.0 | MS, RI, STD | B | |
| 23 | α-Ionone | Pleasant, cream-like, rose-like | 1.7 | 2.5 | MS, RI, STD | B | |
| 24 | trans-β-Ionone | Sweet, cream-like, floral, fruity | 3.0 | 3.0 | 3.0 | MS, RI, STD | B |
| 25 | cis-Calamenene | Fresh, minty, toothpaste-like | 1.7 | 2.2 | MS, RI | A | |
| 26 | Unknown 1 | Green, fresh, grassy | 2.3 | A | |||
| 27 | Unknown 2 | Floral, coffee-like, sweet | 1.8 | 3.0 | B | ||
| 28 | Unknown 3 | Roasted, milky, sweet | 1.8 | 2.0 | C | ||
| 29 | Unknown 4 | Sweet, roasted, floral, burnt | 2.7 | 3.3 | 3.0 | C | |
| 30 | Unknown 5 | Roasted, sweet, cream-like | 2.8 | 2.7 | 3.0 | C | |
| 31 | Unknown 6 | Capsule-like, powder-like | 1.3 | D | |||
| 32 | Unknown 7 | Non-pleasant, irritating | 2.3 | 2.3 | 1.8 | D | |
| 33 | Unknown 8 | Non-pleasant, irritating | 2.6 | 3.0 | 3.3 | D | |
| 34 | Unknown 9 | Stinky | 2.0 | 2.7 | D | ||
| 35 | Unknown 10 | Roasted, coffee-like, burnt | 2.5 | C | |||
| 36 | Unknown 11 | Non-pleasant, plastic-like | 3.0 | 2.0 | D | ||
| 37 | Unknown 12 | Green, fresh, cucumber-like | 2.0 | A | |||
| 38 | Unknown 13 | Non-pleasant, spicy | 2.7 | D | |||
| 39 | Unknown 14 | Non-pleasant, powder-like | 1.7 | 2.5 | D | ||
| 40 | Unknown 15 | Floral, fruity, sweet | 2.0 | B | |||
| 41 | Unknown 16 | Sweet, fruity, minty, citrus | 2.0 | B | |||
| 42 | Unknown 17 | Non-pleasant, irritating | 1.7 | 1.7 | D | ||
| 43 | Unknown 18 | Sweet, caramel-like, floral | 2.3 | 3.3 | 4.0 | B | |
| 44 | Unknown 19 | Sweet, honey, caramel-like | 2.0 | B | |||
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.-C.; Wang, M.-Q.; Ni, D.-J.; Lin, Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules 2020, 25, 6050. https://doi.org/10.3390/molecules25246050
Chen Q-C, Zhu Y, Yan H, Chen M, Xie D-C, Wang M-Q, Ni D-J, Lin Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules. 2020; 25(24):6050. https://doi.org/10.3390/molecules25246050
Chicago/Turabian StyleChen, Qin-Cao, Yin Zhu, Han Yan, Mei Chen, Dong-Chao Xie, Meng-Qi Wang, De-Jiang Ni, and Zhi Lin. 2020. "Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas" Molecules 25, no. 24: 6050. https://doi.org/10.3390/molecules25246050