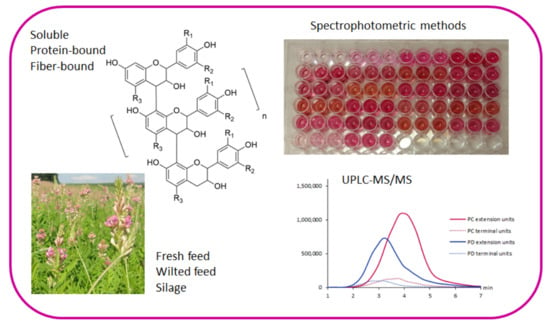
Changes in Feed Proanthocyanidin Profiles during Silage Production and Digestion by Lamb
Abstract
:1. Introduction
2. Results and Discussion
2.1. Soluble and Insoluble PAs by BuOH/HCl/Ace Assay
2.2. The Compositions of Soluble PAs by UPLC-MS/MS
2.3. Soluble, Protein-Bound and Fiber-Bound PAs by Terrill’s Method
2.4. Unidentifiable Role of Insoluble PAs
3. Materials and Methods
3.1. Preparation of the Experimental Forages
3.2. Experimental Lambs and Treatments
3.3. Nutrient Analysis of the Feed
3.4. Extraction of Feed Materials and Digesta Samples for BuOH/HCl/Ace Assays
3.5. Extraction of Feed Materials and Digesta Samples According to Terrill’s Method
3.6. Determination of Contents of Soluble and in-Soluble PAs in the Feed and Digesta Samples by BuOH/HCl/Ace Assay
3.7. Determination of Contents of Soluble and Insoluble PAs in the Feed and Digesta Samples by Terrill’s Method
3.8. UPLC-MS/MS Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.W.F.; Salminen, J.-P.J.-P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop Sci. 2019, 59, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Hemingway, R.W.; Foo, L.Y.; Porter, L.J. Linkage isomerism in trimeric and polymeric 2,3-cis-procyanidins. J. Chem. Soc. Perkin Trans. 1 1982, 1209–1216. [Google Scholar] [CrossRef]
- Porter, L.J. Condensed tannins. In Natural Products of Woody Plants I; Rowe, J.W., Ed.; Springer: Berlin, Germany, 1989; pp. 651–690. [Google Scholar]
- Cheynier, V.; Fulcrand, H. Analysis of polymeric proanthocyanidins and complex polyphenols. In Methods in Polyphenol Analysis; Santos-Buelga, C., Williamson, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J. Procyanidin xylosides from the bark of Betula pendula. Phytochemistry 2012, 76, 178–183. [Google Scholar] [CrossRef]
- Engström, M.T.; Pälijärvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.-P. Rapid qualitative and quantitative analyses of proanthocyanidin oligomers and polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar] [CrossRef]
- Karonen, M.; Leikas, A.; Loponen, J.; Sinkkonen, J.; Ossipov, V.; Pihlaja, K. Reversed-phase HPLC-ESI/MS analysis of birch leaf proanthocyanidins after their acidic degradation in the presence of nucleophiles. Phytochem. Anal. 2007, 18, 378–386. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Tannins of herbaceous leguminosae. Phytochemistry 1973, 12, 1809–1812. [Google Scholar] [CrossRef]
- Lavisci, P.; Scalbert, A.; Masson, D.; Janin, G. Quality of turkey oak (Quercus cerris) wood: Soluble and insoluble proanthocyanidins. Holzforschung 1991, 45, 291–296. [Google Scholar] [CrossRef]
- Matthews, S.; Mila, I.; Scalbert, A.; Donnelly, D.M.X. Extractable and non-extractable proanthocyanidins in barks. Phytochemistry 1997, 45, 405–410. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Nephelometric study of salivary protein-tannin aggregates. J. Sci. Food Agric. 2002, 82, 113–119. [Google Scholar] [CrossRef]
- McManus, J.P.; Davis, K.G.; Beart, J.E.; Gaffney, S.H.; Lilley, T.H.; Haslam, E. Polyphenol interactions. Part 1. Introduction; some observations on the reversible complexation of polyphenols with proteins and polysaccharides. J. Chem. Soc. Perkin Trans. 2 1985, 28, 1429–1438. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Structural features of procyanidin interactions with salivary proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Non-covalent interaction between procyanidins and apple cell wall material: Part I. Effect of some environmental parameters. Biochim. Biophys. Acta 2004, 1672, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-da-Silva, J.M.; Cheynier, V.; Souquet, J.; Moutounet, M.; Cabanis, J.; Bourzeix, M. Interaction of grape seed procyanidins with various proteins in relation to wine fining. J. Sci. Food Agric. 1991, 57, 111–125. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Haemanalysis of tannins: The concept of relative astringency. Phytochemistry 1973, 12, 907–912. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef]
- Grabber, J.H.; Zeller, W.E.; Mueller-Harvey, I. Acetone enhances the direct analysis of procyanidin- and prodelphinidin-based condensed tannins in Lotus species by the butanol-HCl-iron assay. J. Agric. Food Chem. 2013, 61, 2669–2678. [Google Scholar] [CrossRef]
- Rigaud, J.; Perez-Ilzarbe, J.; Ricardo da Silva, J.M.; Cheynier, V. Micro method for the identification of proanthocyanidin using thiolysis monitored by high-performance liquid chromatography. J. Chromatogr. A 1991, 540, 401–405. [Google Scholar] [CrossRef]
- Koupai-Abyazani, M.R.; McCallum, J.; Bohm, B.A. Identification of the constituent flavanoid units in sainfoin proanthocyanidins by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1992, 594, 117–123. [Google Scholar] [CrossRef]
- Hellström, J.K.; Mattila, P.H. HPLC determination of extractable and unextractable proanthocyanidins in plant materials. J. Agric. Food Chem. 2008, 56, 7617–7624. [Google Scholar] [CrossRef] [PubMed]
- Buendía, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F.A. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef] [PubMed]
- Gea, A.; Stringano, E.; Brown, R.H.; Mueller-Harvey, I. In situ analysis and structural elucidation of sainfoin (Onobrychis viciifolia) tannins for high-throughput germplasm screening. J. Agric. Food Chem. 2011, 59, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.-F. Direct thiolysis on crude apple materials for high-performance liquid chromatography characterization and quantification of polyphenols in cider apple tissues and juices. Methods Enzymol. 2001, 335, 57–70. [Google Scholar]
- Matthews, S.; Mila, I.; Scalbert, A.; Pollet, B.; Lapierre, C.; Hervé Du Penhoat, C.L.M.; Rolando, C.; Donnelly, D.M.X. Method for estimation of proanthocyanidins based on their acid depolymerization in the presence of nucleophiles. J. Agric. Food Chem. 1997, 45, 1195–1201. [Google Scholar] [CrossRef]
- Ramsay, A.; Drake, C.; Grosse Brinkhaus, A.; Girard, M.; Copani, G.; Dohme-Meier, F.; Bee, G.; Niderkorn, V.; Mueller-Harvey, I. Sodium Hydroxide Enhances Extractability and Analysis of Proanthocyanidins in Ensiled Sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2015, 63, 9471–9479. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Schreurs, N.M.; McNabb, W.C.; Tavendale, M.H.; Lane, G.A.; Barry, T.N.; Cummings, T.; Fraser, K.; López-Villalobos, N.; Ramírez-Restrepo, C.A. Skatole and indole concentration and the odour of fat from lambs that had grazed perennial ryegrass/white clover pasture or Lotus corniculatus. Anim. Feed Sci. Technol. 2007, 138, 254–271. [Google Scholar] [CrossRef]
- Girard, M.; Dohme-Meier, F.; Wechsler, D.; Goy, D.; Kreuzer, M.; Bee, G. Ability of 3 tanniferous forage legumes to modify quality of milk and Gruyère-type cheese. J. Dairy Sci. 2016, 99, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Girard, M.; Dohme-Meier, F.; Silacci, P.; Ampuero Kragten, S.; Kreuzer, M.; Bee, G. Forage legumes rich in condensed tannins may increase n-3 fatty acid levels and sensory quality of lamb meat. J. Sci. Food Agric. 2016, 96, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Girard, M. Bioactive Compounds in Forage Legumes: Structural Changes during Conservation, Their Fate along the Digestive Tract and Their Potential to Impact Ruminant Products; ETH Zurich: Zürich, Switzerland, 2016. [Google Scholar]
- Terrill, T.H.; Rowan, A.H.; Douglas, G.B.; Barry, T. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Soliva, C.R.; Wyss, U.; Kreuzer, M.; Dohme, F. Palatability in sheep and invitro nutritional value of dried and ensiled sainfoin (Onobrychis viciifolia) birdsfoot trefoil (Lotus corniculatus ), and chicory (Cichorium intybus). Arch. Anim. Nutr. 2007, 61, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Dohme-Meier, F.; Kragten, S.; Brinkhaus, A.; Arrigo, Y.; Wyss, U.; Bee, G. Modification of the proportion of extractable and bound condensed tannins in birdsfoot trefoil (Lotus corniculatus) and sainfoin (Onobrychis viicifolia) during wilting, ensiling and pelleting processes. Biotechnol. Anim. Husb. 2018, 34, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.M.; Eriksson, T.; Udén, P. Effect of wilting, silage additive, PEG treatment and tannin content on the distribution of N between different fractions after ensiling of three different sainfoin (Onobrychis viciifolia) varieties. Grass Forage Sci. 2010, 65, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Minnee, E.M.K.; Woodward, S.L.; Waghom, G.C.; Laboyrie, P.G. The effect of ensiling forage legumes on condensed tannins. Agron. N. Z. 2002, 32/33, 117–119. [Google Scholar]
- Terrill, T.H.; Waghorn, G.C.; Woolley, D.J.; Mcnabb, W.C.; Barry, T.N. Assay and digestion of 14C-labelled condensed tannins in the gastrointestinal tract of sheep. Br. J. Nutr. 1994, 72, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Ropiak, H.M.; Lachmann, P.; Ramsay, A.; Green, R.J.; Mueller-Harvey, I. Identification of structural features of condensed tannins that affect protein aggregation. PLoS ONE 2017, 12, e0170768. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Effect of tannin structure. Encycl. Food Chem. 2019, 2, 515–521. [Google Scholar]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Kariuki, I.W.; Norton, B.W. The digestion of dietary protein bound by condensed tannins in the gastro-intestinal tract of sheep. Anim. Feed Sci. Technol. 2008, 142, 197–209. [Google Scholar] [CrossRef]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Maldonado, R.A.; Norton, B.W. Digestion of 14C-labelled condensed tannins from Desmodium intortum in sheep and goats. Br. J. Nutr. 1996, 76, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quijada, J.; Drake, C.; Gaudin, E.; El-Korso, R.; Hoste, H.; Mueller-Harvey, I. Condensed tannin changes along the digestive tract in lambs fed with sainfoin pellets or hazelnut skins. J. Agric. Food Chem. 2018, 66, 2136–2142. [Google Scholar] [CrossRef]
- Desrues, O.; Mueller-Harvey, I.; Pellikaan, W.F.; Enemark, H.L.; Thamsborg, S.M. Condensed tannins in the gastrointestinal tract of cattle after sainfoin (Onobrychis viciifolia) intake and their possible relationship with anthelmintic effects. J. Agric. Food Chem. 2017, 65, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Bruni, R.; Del Rio, D. Bioactivation of High-Molecular-Weight Polyphenols by the Gut Microbiome; Rio, K.T.D., Ed.; Academic Press: San Diego, FL, USA, 2015; ISBN 9780124079410. [Google Scholar]





| DM | OM | CP | NDF | ADF | |
|---|---|---|---|---|---|
| Fresh | |||||
| Sainfoin | 173 | 915 | 137 | 398 | 387 |
| Birdsfoot trefoil | 161 | 903 | 204 | 397 | 306 |
| Wilted | |||||
| Sainfoin | 408 | 900 | 132 | 419 | 373 |
| Birdsfoot trefoil | 337 | 894 | 200 | 443 | 348 |
| Silage | |||||
| Sainfoin | 365 | 911 | 138 | 459 | 401 |
| Birdsfoot trefoil | 363 | 879 | 189 | 484 | 351 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, M.; Lehtimäki, A.; Bee, G.; Dohme-Meier, F.; Karonen, M.; Salminen, J.-P. Changes in Feed Proanthocyanidin Profiles during Silage Production and Digestion by Lamb. Molecules 2020, 25, 5887. https://doi.org/10.3390/molecules25245887
Girard M, Lehtimäki A, Bee G, Dohme-Meier F, Karonen M, Salminen J-P. Changes in Feed Proanthocyanidin Profiles during Silage Production and Digestion by Lamb. Molecules. 2020; 25(24):5887. https://doi.org/10.3390/molecules25245887
Chicago/Turabian StyleGirard, Marion, Annika Lehtimäki, Giuseppe Bee, Frigga Dohme-Meier, Maarit Karonen, and Juha-Pekka Salminen. 2020. "Changes in Feed Proanthocyanidin Profiles during Silage Production and Digestion by Lamb" Molecules 25, no. 24: 5887. https://doi.org/10.3390/molecules25245887