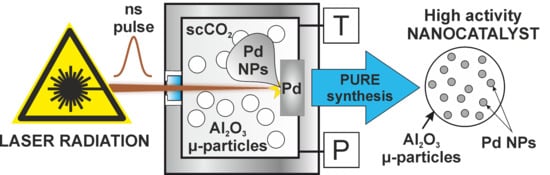
Synthesis of Supported Heterogeneous Catalysts by Laser Ablation of Metallic Palladium in Supercritical Carbon Dioxide Medium
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Installation
3.2. Materials
3.3. Laser Exposition
3.4. Methods of Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Türk, M.; Erkey, C. Synthesis of supported nanoparticles in supercritical fluids by supercritical fluid reactive deposition: Current state, further perspectives and needs. J. Supercrit. Fluids 2018, 134, 176–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Erkey, C. Preparation of supported metallic nanoparticles using supercritical fluids: A review. J. Supercrit. Fluids 2006, 38, 252–267. [Google Scholar] [CrossRef]
- Deal, J.W.; Le, P.; Corey, C.B.; More, K.; West, C.W. Water-gas shift reaction on alumina-supported Pt-CeOx catalysts prepared by supercritical fluid deposition. J. Supercrit. Fluids 2017, 119, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Ulusal, F.; Darendeli, B.; Erünal, E.; Egitmen, A.; Guzel, B. Supercritical carbondioxide deposition of Γ-Alumina supported Pd nanocatalysts with new precursors and using on Suzuki-Miyaura coupling reactions. J. Supercrit. Fluids 2017, 127, 111–120. [Google Scholar] [CrossRef]
- Morre, J.; Tenorio, M.J.; Torralvo, M.J.; Pando, C.; Renuncio, J.A.R.; Cabañas, A. Deposition of Pd into mesoporous silica SBA-15 using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 56, 213–222. [Google Scholar] [CrossRef]
- Hunt, A.J.; Budarin, V.L.; Comerford, J.W.; Parker, H.L.; Lazarov, V.K.; Breeden, S.W.; Macquarrie, D.J.; Clark, J.H. Deposition of palladium nanoparticles in SBA-15 templated silica using supercritical carbon dioxide. Mater. Lett. 2014, 116, 408–411. [Google Scholar] [CrossRef]
- Ushiki, I.; Takahashi, N.; Shimizu, T.; Sato, Y.; Ota, M.; Smith, R.L.; Inomata, H. Adsorption equilibria of rhodium acetylacetonate with MCM-41, MSU-H, and HMS silica substrates in supercritical carbon dioxide for preparing catalytic mesoporous materials. J. Supercrit. Fluids 2017, 120, 240–248. [Google Scholar] [CrossRef]
- Cangül, B.; Zhang, L.C.; Aindow, M.; Erkey, C. Preparation of carbon black supported Pd, Pt and Pd-Pt nanoparticles using supercritical CO2 deposition. J. Supercrit. Fluids 2009, 50, 82–90. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, H.; Liu, Z.; Ji, M.; Zhang, L.; Sun, G. Supercritical deposition route of preparing pt/graphene composites and their catalytic performance toward methanol electrooxidation. J. Phys. Chem. C 2014, 118, 1182–1190. [Google Scholar] [CrossRef]
- Morère, J.; Sánchez-Miguel, E.; Tenorio, M.J.; Pando, C.; Cabañas, A. Supercritical fluid preparation of Pt, Ru and Ni/graphene nanocomposites and their application as selective catalysts in the partial hydrogenation of limonene. J. Supercrit. Fluids 2017, 120, 7–17. [Google Scholar] [CrossRef]
- Pan, H.B.; Yen, C.H.; Yoon, B.; Sato, M.; Wai, C.M. Recyclable and ligandless suzuki coupling catalyzed by carbon nanotube-supported palladium nanoparticles synthesized in supercritical fluid. Synth. Commun. 2006, 36, 3473–3478. [Google Scholar] [CrossRef]
- Yen, C.H.; Shimizu, K.; Lin, Y.Y.; Bailey, F.; Cheng, I.F.; Wai, C.M. Chemical fluid deposition of Pt-based bimetallic nanoparticles on multiwalled carbon nanotubes for direct methanol fuel cell application. Energy Fuels 2007, 21, 2268–2271. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, D.; Aindow, M.; Erkey, C. Preparation and characterization of ruthenium/carbon aerogel nanocomposites via a supercritical fluid route. J. Phys. Chem. B 2005, 109, 2617–2624. [Google Scholar] [CrossRef]
- Saquing, C.D.; Kang, D.; Aindow, M.; Erkey, C. Investigation of the supercritical deposition of platinum nanoparticles into carbon aerogels. Microporous Mesoporous Mater. 2005, 80, 11–23. [Google Scholar] [CrossRef]
- Watkins, J.J.; McCarthy, T.J. Polymer/Metal Nanocomposite Synthesis in Supercritical CO2. Chem. Mater. 1995, 7, 1991–1994. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, C.; Liu, Z.; Han, B.; Xie, Y.; Ding, S.; Yang, Z. Highly efficient nanocatalysts supported on hollow polymer nanospheres: Synthesis, characterization, and applications. J. Phys. Chem. C 2008, 112, 774–780. [Google Scholar] [CrossRef]
- Parenago, O.P.; Timashev, P.S.; Karakhanov, E.A.; Maximov, A.L.; Lazhko, A.E.; Zolotukhina, A.V.; Bagratashvili, V.N. Obtaining of highly-active catalysts of unsaturated compounds hydrogenation by using supercritical carbon dioxide. J. Supercrit. Fluids 2018, 140, 387–393. [Google Scholar] [CrossRef]
- Zhang, J.; Chaker, M.; Ma, D. Pulsed laser ablation based synthesis of colloidal metal nanoparticles for catalytic applications. J. Colloid Interface Sci. 2017, 489, 138–149. [Google Scholar] [CrossRef]
- Yang, G. Laser Ablation in Liquids: Principles and Applications in the Preparation of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9814241520. [Google Scholar]
- Yang, D. Applications of Laser Ablation—Thin Film Deposition, Nanomaterial Synthesis and Surface Modification; Yang, D., Ed.; InTech: Rijeka, Croatia, 2016; ISBN 9535128116. [Google Scholar]
- Ma, R.; Kim, Y.J.; Amaranatha Reddy, D.; Kim, T.K. Synthesis of CeO2/Pd nanocomposites by pulsed laser ablation in liquids for the reduction of 4-nitrophenol to 4-aminophenol. Ceram. Int. 2015, 41, 12432–12438. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ma, R.; Reddy, D.A.; Kim, T.K. Liquid-phase pulsed laser ablation synthesis of graphitized carbon-encapsulated palladium core-shell nanospheres for catalytic reduction of nitrobenzene to aniline. Appl. Surf. Sci. 2015, 357, 2112–2120. [Google Scholar] [CrossRef]
- Park, H.; Reddy, D.A.; Kim, Y.; Lee, S.; Ma, R.; Lim, M.; Kim, T.K. Hydrogenation of 4-nitrophenol to 4-aminophenol at room temperature: Boosting palladium nanocrystals efficiency by coupling with copper via liquid phase pulsed laser ablation. Appl. Surf. Sci. 2017, 401, 314–322. [Google Scholar] [CrossRef]
- Stauss, S.; Urabe, K.; Muneoka, H.; Terashima, K. Pulsed Laser Ablation in High-Pressure Gases, Pressurized Liquids and Supercritical Fluids: Generation, Fundamental Characteristics and Applications. In Applications of Laser Ablation—Thin Film Deposition, Nanomaterial Synthesis and Surface Modification; Yang, D., Ed.; InTech: Rijeka, Croatia, 2016; p. 221. ISBN 9535128116. [Google Scholar]
- Saitow, K. Silicon Nanoclusters Selectively Generated by Laser Ablation in Supercritical Fluid. J. Phys. Chem. B 2005, 109, 3731–3733. [Google Scholar] [CrossRef]
- Nakahara, S.; Stauss, S.; Kato, T.; Sasaki, T.; Terashima, K. Synthesis of higher diamondoids by pulsed laser ablation plasmas in supercritical CO2. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono; Takada, N.; Kanda, H.; Sasaki, K.; Goto, M. Fabrication of gold and silver nanoparticles with pulsed laser ablation under pressurized CO2. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Minaev, N.V.; Arakcheev, V.G.; Rybaltovskii, A.O.; Firsov, V.V.; Bagratashvili, V.N. Dynamics of formation and decay of supercritical fluid silver colloid under pulse laser ablation conditions. Russ. J. Phys. Chem. B 2015, 9, 1074–1081. [Google Scholar] [CrossRef]
- Tsypina, S.I.; Epifanov, E.O.; Shubny, A.G.; Arakcheev, V.G.; Minaev, N.V.; Rybaltovskii, A.O. Single-Stage Formation of Film Polymer Composites in Supercritical Colloid Solutions of Nanoparticles Obtained by Laser Ablation. Russ. J. Phys. Chem. B 2019, 13, 1235–1244. [Google Scholar] [CrossRef]
- Singh, A.; Salminen, T.; Honkanen, M.; Nikkanen, J.P.; Vuorinen, T.; Kari, R.; Vihinen, J.; Levänen, E. Carbon coated TiO2 nanoparticles prepared by pulsed laser ablation in liquid, gaseous and supercritical CO2. Nanotechnology 2020, 31. [Google Scholar] [CrossRef]
- Singh, A.; Salminen, T.; Honkanen, M.; Vihinen, J.; Hyvärinen, L.; Levänen, E. Multiphase Ti x O y nanoparticles by pulsed laser ablation of titanium in supercritical CO 2. Appl. Surf. Sci. 2019, 476, 822–827. [Google Scholar] [CrossRef]
- Epiphanov, E.O.; Shubnyi, A.G.; Minayev, N.V.; Rybaltovskii, A.O.; Uusupop, V.I.; Parenago, O.P. Applied the Synthesis of Heterogeneous Catalysts by Laser Ablation of Metallic Palladium with Pdeposition on Alumina in the Supercritical Carbon Dioxid. Sverhkriticheskie Flyuidy Teor. i Prakt. 2019, 14, 64–70. [Google Scholar] [CrossRef]
- Xie, X.Z.; Pan, Z.Y.; Wei, X.; Huang, F.M.; Hu, W.; Hong, M.H. Hybrid micromachining of transparent sapphire substrate by pulsed green laser irradiation. J. Laser Micro Nanoeng. 2011, 6, 209–213. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Tkachenko, O.P.; Mashkovsky, I.S.; Yakushev, I.A.; Kozitsyna, N.Y.; Vargaftik, M.N.; Stakheev, A.Y. Pd–Cu catalysts from acetate complexes in liquid-phase diphenylacetylene hydrogenation. Kinet. Catal. 2015, 56, 591–597. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Mashkovsky, I.S.; Stakheev, A.Y. Highly Selective Pd–Cu/α-Al2O3 Catalysts for Liquid-Phase Hydrogenation: The Influence of the Pd: Cu Ratio on the Structure and Catalytic Characteristics. Kinet. Catal. 2018, 59, 601–609. [Google Scholar] [CrossRef]
- Lazhko, A.E.; Bragina, G.O.; Lyubimov, S.E.; Davankov, V.A.; Staheev, A.Y.; Parenago, O.P. Synthesis of Polymer—Metal Composites by Impregnation of Super-Crosslinked Polystyrene with Palladium Compounds in Supercritical Carbon Dioxide and their Catalytic Activity in Liquid-Phase Hydrogenation of Diphenylacetylene. Sverhkriticheskie Flyuidy Teor. i Prakt. 2019, 14, 63–71. [Google Scholar] [CrossRef]







| No. | Medium Density, g/cm3 (T, °C; P, atm) | Ablation Time, min | Time of Ablation with a Stirrer, min | Wavelength λ, nm (Pulse Energy, mJ) | Pd Content, wt % | Proportion of Pd in Al2O3 Fraction > 0.25 mm, % |
|---|---|---|---|---|---|---|
| 1 | 0.2 (70; 100) | 10 | 10 | 1064 (270) | 0.45 | 30 |
| 2 | 0.4 (70; 130) | 10 | 10 | 1064 (270) | 0.19 | 61 |
| 3 | 0.8 (50; 210) | 10 | 10 | 1064 (270) | 0.20 | 92 |
| 4 | 0.8 (50; 210) | 15 | 15 | 532 (110) | 0.35 | - |
| Al2O3 Fractions, mm | Initial (Sample 5) | Fraction 0.25–0.5 (Sample 5-1) | Fraction 0.1–0.25 (Sample 5-2) | Fraction Less Than 0.1 |
|---|---|---|---|---|
| Mass of fraction, g | 1.5 | 1.0 | 0.3 | 0.2 |
| Pd content, wt % | 0 | 0.1 | 0.47 | Not determined |
| Fraction # | Fraction Size, mm | Pd Content, wt % | Wobserv. × 105, mol/min | TOF, s−1 | ||
|---|---|---|---|---|---|---|
| 1st Stage | 2nd Stage | 1st Stage | 2nd Stage | |||
| 5-1 | 0.25–0.5 | 0.1 | 0.60 ± 0.15 | 0.08 ± 0.02 | 2.11 ± 0.2 | 0.28 ± 0.05 |
| 5-2 | 0.1–0.5 | 0.47 | 1.60 ± 0.20 | 0.15 ± 0.02 | 1.20 ± 0.15 | 0.12 ± 0.04 |
Sample Availability: Samples of the Al2O3:PdNP catalyst are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parenago, O.; Rybaltovsky, A.; Epifanov, E.; Shubnyi, A.; Bragina, G.; Lazhko, A.; Khmelenin, D.; Yusupov, V.; Minaev, N. Synthesis of Supported Heterogeneous Catalysts by Laser Ablation of Metallic Palladium in Supercritical Carbon Dioxide Medium. Molecules 2020, 25, 5807. https://doi.org/10.3390/molecules25245807
Parenago O, Rybaltovsky A, Epifanov E, Shubnyi A, Bragina G, Lazhko A, Khmelenin D, Yusupov V, Minaev N. Synthesis of Supported Heterogeneous Catalysts by Laser Ablation of Metallic Palladium in Supercritical Carbon Dioxide Medium. Molecules. 2020; 25(24):5807. https://doi.org/10.3390/molecules25245807
Chicago/Turabian StyleParenago, Oleg, Alexey Rybaltovsky, Evgeniy Epifanov, Andrey Shubnyi, Galina Bragina, Alexey Lazhko, Dmitry Khmelenin, Vladimir Yusupov, and Nikita Minaev. 2020. "Synthesis of Supported Heterogeneous Catalysts by Laser Ablation of Metallic Palladium in Supercritical Carbon Dioxide Medium" Molecules 25, no. 24: 5807. https://doi.org/10.3390/molecules25245807