Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of COEO Inclusion Complex
2.1.1. Recovery of COEO Inclusion Complex
2.1.2. Entrapment Efficiency (%EE)
2.1.3. Particle Size and Morphology
2.1.4. FT-IR Analysis
2.2. Biological Assay
2.2.1. Antioxidant Activity
2.2.2. Antifungal Activity
2.2.3. Antibacterial Activity
3. Materials and Methods
3.1. Materials
Concentrated Orange Oils
3.2. Formation and Characterization of the Inclusion Complex
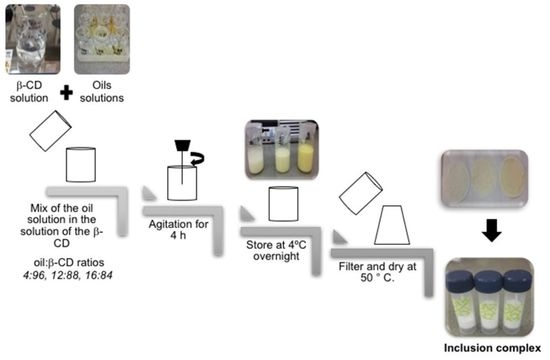
3.2.1. Preparation of β-Cyclodextrin Inclusion Complexes
3.2.2. Entrapment Efficiency (%EE)
3.2.3. Particle Size and Morphology
3.2.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
3.3. Biological Assay
3.3.1. Antioxidant Activity Assay
3.3.2. Antifungal Assay
3.3.3. Antibacterial Activity
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galvão, J.G.; Silva, V.F.; Ferreira, S.G.; França, F.R.M.; Santos, D.A.; Freitas, L.S.; Alves, P.B.; Araújo, A.A.S.; Cavalcanti, S.C.H.; Nunes, R.S. β–cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochim. Acta 2015, 608, 14–19. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Campbell, H.E.; García, C.; Coronado, M.A.; León, J.A.; Sagaste, C.A.; Pérez, L.J. Extraction and Characterization of Orange Peel Essential Oil from Mexico and United States of America. J. Essent. Oil Bear. Plants 2017, 20, 897–914. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Essential oil in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Velázquez-Nuñez, M.J.; Avila-Sosa, R.; Palou, E.; López-Malo, A. Antifungal activity orange (Citrus sinensis var. Valencia) peel essential oil applied by direct addition or vapor contact. Food Control 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Xu, B.M.; Baker, G.L.; Sarnoski, P.J.; Goodrich-Schneider, R.M. A Comparison of the Volatile Components of Cold Pressed Hamlin and Valencia (Citrus sinensis (L.) Osbeck) Orange Oils Affected by Huanglongbing. J. Food Qual. 2017, 1–20. [Google Scholar] [CrossRef]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Dias, A.R.G.; Zavareze, E.D.R. Production, characterization, and stability of orange or eucalyptus essential oil/β–cyclodextrin inclusion complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.R.; Pauletti, G.F. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Beneti, S.C.; Rosset, E.; Corazza, M.L.; Frizzo, C.D.; Di Luccio, M.; Oliveira, J.V. Fractionation of citronella (Cymbopogon winterianus) essential oil and concentrated orange oil phase by batch vacuum distilation. J. Food Eng. 2011, 102, 348–354. [Google Scholar] [CrossRef]
- Lopes, D.; Raga, A.C.; Stuart, G.R.; de Oliveira, J.V. Influence of vacuum distillation parameters on the chemical composition of a five-fold sweet orange oil (Citrus sinensis Osbeck). J. Essent. Oil Res. 2003, 15, 408–411. [Google Scholar] [CrossRef]
- López-Muñoz, G.A.; Balderas-López, J.A. Photothermal characterization of citrus essential oils and their derivatives. Thermochim. Acta 2014, 579, 40–44. [Google Scholar] [CrossRef]
- Torres-Alvarez, C.; Núñez, A.G.; Rodríguez, J.; Castillo, S.; Leos-Rivas, C.; Baéz-González, J.G. Chemical composition, antimicrobial, and antioxidant activities of orange essential oil and its concentrated oils. CyTA J. Food 2017, 15, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Barba, C.; Eguinoa, A.; Maté, J.I. Preparation and characterization of β-cyclodextrin inclusion complexes as a tool of a controlled antimicrobial release in whey protein edible films. LWT-Food Sci. Technol. 2015, 64, 1362–1369. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, physicochemical characterization and release behavior of the inclusion complex of trans-anethole and β–cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef]
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Complexation of estragole as pure compound and as main component of basil and tarragon essential oils with cyclodextrins. Carbohydr. Polym. 2015, 118, 156–164. [Google Scholar] [CrossRef]
- Angelova, A.; Ringard-Lefebvre, C.; Baszkin, A. Drug–Cyclodextrin Association Constants Determined by Surface Tension and Surface Pressure Measurements: I. Host–Guest Complexation of Water Soluble Drugs by Cyclodextrins: Polymyxin B–β Cyclodextrin System. J Colloid Interface Sci. 1999, 212, 275–279. [Google Scholar] [CrossRef]
- Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Lacovino, R. Alpha- and β-cyclodextrin inclusion complexes with 5-fluorouracil: Characterization and cytotoxic activity evaluation. Molecules 2016, 21, 1644. [Google Scholar] [CrossRef]
- Angelova, A.; Ringard-Lefebvre, C.; Baszkin, A. Drug–Cyclodextrin Association Constants Determined by Surface Tension and Surface Pressure Measurements: II. Sequestration of Water Insoluble Drugs from the Air–Water Interface: Retinol–β Cyclodextrin System. J. Colloid Interface Sci. 1999, 212, 280–285. [Google Scholar] [CrossRef]
- Zerkoune, L.; Lesieur, S.; Putaux, J.; Choisnard, L.; Gèze, A.; Wouessidjewe, D.; Angelov, B.; Vebert-Nardin, C.; Doutch, J.; Angelova, A. Mesoporous self-assembled nanoparticles of biotransesterified cyclodextrins and nonlamellar lipids as carriers of water-insoluble substances. Soft Matter. 2016, 12, 7539–7550. [Google Scholar] [CrossRef] [Green Version]
- Zerkoune, L.; Angelova, A.; Lesieur, S. Review Nano-Assemblies of modified cyclodextrins and their complexes with guest molecules: Incorporation in nanostructured membranes and amphiphile nanoarchitectonics design. Nanomaterials 2014, 4, 741–765. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res. Int. 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β–cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Samperio, C.; Boyer, R.; Eigel, W.N., 3rd; Holland, K.W.; McKinney, J.S.; O’Keefe, S.F.; Smith, R.; Marcy, J.E. Enhancement of plant essential oils’ aqueous solubility and stability using alpha and β cyclodextrin. J. Agric. Food Chem. 2010, 58, 12950–12956. [Google Scholar] [CrossRef]
- Del Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.A.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Controlled release of antifungal volatiles of thyme essential oil from β–cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Dos Passos Menezes, P.; Dos Santos, P.B.P.; Dória, G.A.A.; Sousa, B.M.H.; Serafini, M.R.; Nunes, P.S.; Quintans-Júnior, L.J.; Matos, I.L.; Alves, P.B.; Bezerra, D.P.; et al. Molecular modeling and physicochemical properties of supramolecular complexes of limonene with α– and β–cyclodextrins. AAPS Pharm. Sci. Tech. 2017, 18, 49–57. [Google Scholar] [CrossRef]
- Menezes, P.P.; Serafini, M.R.; Quintans-Júnior, L.J.; Silva, G.F.; Oliveira, J.F.; Carvalho, F.M.S.; Souza, J.C.C.; Matos, J.R.; Alves, P.B.; Matos, I.L.; et al. Inclusion complex of (−)-linalool and β-cyclodextrin. J. Therm. Anal. Calorim. 2014, 115, 2429–2437. [Google Scholar] [CrossRef]
- Songkro, S.; Hayook, N.; Jaisawang, J.; Maneenuan, D.; Chuchome, T.; Kaewnopparat, N. Investigation of inclusion complexes of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 339–355. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of chlorogenic acid with β–cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocoll. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Ferreira, F.D.R.; Valentim, I.B.; Ramones, E.L.C.; Trevisan, M.T.S.; Olea-Azar, C.; Perez-Cruz, F.; de Abreu, F.C.; Goulart, M.O.F. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT-Food Sci. Technol. 2013, 51, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Zavala, J.F.; Soto-Valdez, H.; González-León, A.; Álvarez-Parrilla, E.; Martín-Belloso, O.; González-Aguilar, G.A. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β–cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2008, 60, 359–368. [Google Scholar] [CrossRef]
- Petrović, G.M.; Stojanović, G.S.; Radulović, N.S. Encapsulation of cinnamon oil in β–cyclodextrin. J. Med. Plants Res. 2010, 4, 1382–1390. [Google Scholar] [CrossRef]
- Haloci, E.; Toska, V.; Shkreli, R.; Goci, E.; Vertuani, S.; Manfredini, S. Encapsulation of Satureja montana essential oil in β–cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 147–153. [Google Scholar] [CrossRef]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol β-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT-Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-β-cyclodextrin. LWT-Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and characterization of β–cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT-Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Eun-Ju, S.; Sang-Gi, M.; Mi-Jung, C. Release characteristics of freeze-dried eugenol encapsulated with β-cyclodextrin by molecular inclusion method. J. Microencapsul. 2010, 27, 496–505. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of β-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT-Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Oliveira, M.A.; Alves Rdos, S.; Menezes Pdos, P.; Serafini, M.R.; Araujo, A.A.S.; Pereira, D.B.; Quintans Júnior, L.J. Encapsulation of carvacrol, a monoterpene present in the essential oil of oregano, with β–cyclodextrin, improves the pharmacological response on cancer pain experimental protocols. Chem. Biol. Interact. 2015, 227, 69–76. [Google Scholar] [CrossRef]
- Duarte, A.; Martinho, A.; Luís, Â.; Figueiras, A.; Oleastro, M.; Domingues, F.C.; Silva, F. Resveratrol encapsulation with methyl-β–cyclodextrin for antibacterial and antioxidant delivery applications. LWT-Food Sci. Technol. 2015, 63, 1254–1260. [Google Scholar] [CrossRef]
- Mayaud, L.; Carricajo, A.; Zhiri, A.; Aubert, G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett. Appl. Microbiol. 2008, 47, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Carazo-Díaz, C.; Gabaldón, J.A.; Núñez-Delicado, E. Optimization of the microencapsulation of synthetic strawberry flavour with different blends of encapsulating agents using spray drying. Powder Technol. 2018, 338, 591–598. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rosas-Taraco, A.; Sánchez, E.; García, S.; Heredia, N.; Bhatnagar, D. Extracts of Agave americana inhibit aflatoxin production in Aspergillus parasiticus. World Mycotoxin J. 2011, 4, 37–42. [Google Scholar] [CrossRef]
- Sánchez, E.; Heredia, N.; García, S. Inhibition of growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. Int. J. Food Microbiol. 2005, 98, 271–279. [Google Scholar] [CrossRef]
- Castillo, S.L.; Heredia, N.; Contreras, J.F.; García, S. Extracts of edible and medicinal plants in inhibition of growth, adherence, and cytotoxin production of Campylobacter jejuni and Campylobacter coli. J. Food Sci. 2011, 76, 421–426. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Meth. 2010, 81, 121–126. [Google Scholar] [CrossRef]





| COEO: β–CD Ratio | Recovery (%) * | ||
|---|---|---|---|
| 5x | 10x | 20x | |
| 4:96 | 78.4 ± 0.7 b,c | 79.00 ± 1.9 b,c | 74.4 ± 2.2 c |
| 12:88 | 83.3 ± 1.1 a,b | 81.2 ± 2.3 a,b | 80.9 ± 1.7 a,b |
| 16:84 | 84.5 ± 2.2 a | 84.8 ± 1.1 a | 82.0 ± 2.1 a,b |
| COEO | Ratio | Encapsulated Oil * (mg/g β–CD) | Entrapment Efficiency (%) |
|---|---|---|---|
| 5x | 4:96 | 7.8 ± 1.3 f | 75.6 ± 0.1 c |
| 12:88 | 23.7 ± 1.6 e | 84.4 ± 3.0 a,b | |
| 16:84 | 35.8 ± 4.1 d | 85.2 ± 2.4 ab | |
| 10x | 4:96 | 7.7 ± 1.3 f | 84.5 ± 2.0 ab |
| 12:88 | 58.1 ± 1.0 c | 80.7 ± 3.4 b,c | |
| 16:84 | 35.9 ± 2.2 d | 67.1 ± 3.4 d | |
| 20x | 4:96 | 18.2 ± 2.5 e | 85.8 ± 0.9 a,b |
| 12:88 | 102.3 ± 3.4 a | 89.5 ± 0.4 a | |
| 16:84 | 83.1 ± 1.5 b | 74.5 ± 1.4 c |
| COEO: β–CD Ratio | Particle Size (μm) * | ||
|---|---|---|---|
| 5x | 10x | 20x | |
| 4:96 | 3.59 ± 0.03 a | 2.11 ± 0.02 a | 3.64 ± 0.7 a |
| 12:88 | 2.46 ± 0.01 b | 1.62 ± 0.01 c | 1.51 ± 0.01 c |
| 16:84 | 1.97 ± 0.01 c | 1.99 ± 0.07 b | 1.90 ± 0.01 b |
| Fungus | Ratio | COEO | mg/mL | Control | |||
|---|---|---|---|---|---|---|---|
| 1.25 | 2.5 | 5 | 10 | ||||
| Aspergillus niger | 12:88 | 5x | +++ | +++ | +++ | ++ | +++ |
| 10x | +++ | ++ | ++ | + | +++ | ||
| 20x | +++ | +++ | +++ | +++ | +++ | ||
| 16:84 | 5x | +++ | ++ | ++ | ++ | +++ | |
| 10x | +++ | ++ | + | + | +++ | ||
| 20x | +++ | +++ | +++ | +++ | +++ | ||
| not encapsulated | 5x | +++ | +++ | ++ | - | +++ | |
| 10x | ++ | ++ | + | - | +++ | ||
| 20x | +++ | +++ | + | - | +++ | ||
| Aspergillus flavus | 12:88 | 5x | +++ | +++ | +++ | ++ | +++ |
| 10x | +++ | +++ | +++ | ++ | +++ | ||
| 20x | +++ | +++ | +++ | +++ | +++ | ||
| 16:84 | 5x | +++ | +++ | +++ | ++ | +++ | |
| 10x | +++ | +++ | +++ | ++ | +++ | ||
| 20x | +++ | +++ | +++ | ++ | +++ | ||
| not encapsulated | 5x | +++ | +++ | ++ | - | +++ | |
| 10x | +++ | +++ | + | + | +++ | ||
| 20x | +++ | +++ | ++ | - | +++ | ||
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Alvarez, C.; Castillo, S.; Sánchez-García, E.; Aguilera González, C.; Galindo-Rodríguez, S.A.; Gabaldón-Hernández, J.A.; Báez-González, J.G. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules 2020, 25, 5109. https://doi.org/10.3390/molecules25215109
Torres-Alvarez C, Castillo S, Sánchez-García E, Aguilera González C, Galindo-Rodríguez SA, Gabaldón-Hernández JA, Báez-González JG. Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations. Molecules. 2020; 25(21):5109. https://doi.org/10.3390/molecules25215109
Chicago/Turabian StyleTorres-Alvarez, Cynthia, Sandra Castillo, Eduardo Sánchez-García, Carlos Aguilera González, Sergio Arturo Galindo-Rodríguez, José A. Gabaldón-Hernández, and Juan G. Báez-González. 2020. "Inclusion Complexes of Concentrated Orange Oils and β-Cyclodextrin: Physicochemical and Biological Characterizations" Molecules 25, no. 21: 5109. https://doi.org/10.3390/molecules25215109