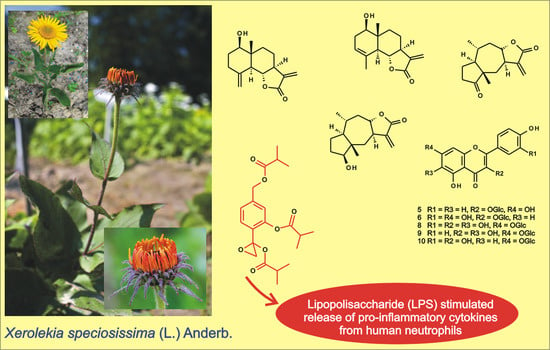
Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate
Abstract
:1. Introduction
2. Results
2.1. Phenolic Compounds in Roots and Aerial Parts of X. speciosissima
2.2. Caffeic Acid Derivatives in Leaves of X. speciosissima, Buphthalmum salicifolium and Telekia speciosa
2.3. Constituents of a Hydroalcoholic Extract from Capitula of X. speciosissima
2.4. Constituents of a Chloroform Extract from Aerial Parts of X. speciosissima
2.5. Effects of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate on Lipopolysaccharide (LPS)-Stimulated Release of Pro-Inflammatory Cytokines From Human Neutrophils
2.5.1. Cytotoxicity
2.5.2. Reactive Oxygen Species (ROS) Generation
2.5.3. Release of Selected Pro-Inflammatory Cytokines/Chemokines (IL-8, TNF-A, IL-1β, CCL2)
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Chemicals and Solvents
4.3. Plant Material
4.4. Estimation of Total Phenolic Content (TPC)
4.5. Phenolic Compounds Analysis by HPLC
4.5.1. Preparation of Samples for HPLC-DAD and UHPLC-DAD-MSn Analysis
4.5.2. Characterization of X. speciosissima Shoot and Root Extracts by UHPLC-DAD-MSn Method
4.5.3. Qualitative Analysis of X. speciosissima, T. speciosa and B. salicifolium Leaf Extracts and Quantification of Commonly Distributed Caffeic Acid Derivatives by HPLC-DAD
4.6. Isolation of Chemical Constituents from a Hydroalcoholic Extract of X. speciosissima Inflorescences
4.7. Isolation of Chemical Constituents from a Chloroform Extract of X. speciosissima Aerial Parts
4.8. Assessment of the Effects of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate on Lipopolysaccharide (LPS)-Stimulated Release of Pro-Inflammatory Cytokines from Human Neutrophils
4.8.1. Isolation of Human Neutrophils
4.8.2. Cytotoxicity Measurement
4.8.3. ROS Production by Neutrophils
4.8.4. IL-8, IL-1β, CCL-2 and TNFα Production by Neutrophils
4.8.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Anderberg, A.A. Taxonomy and phylogeny of the tribe Inuleae (Asteraceae). Plant Syst. Evol. 1991, 176, 75–123. [Google Scholar] [CrossRef]
- Englund, M.; Pornpongrungrueng, P.; Gustafsson, M.H.G.; Anderberg, A.A. Phylogenetic relationships and generic delimitation in Inuleae subtribe Inulinae (Asteraceae) based on ITS and cpDNA sequence data. Cladistics 2009, 25, 319–352. [Google Scholar] [CrossRef]
- Brusa, G.; Ceriani, R.; Cerabolini, B. Seed germination in a narrow endemic species (Telekia speciosissima, Asteraceae): Implications forex situconservation. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2007, 141, 56–61. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-P.; Wang, G.-W.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Kłeczek, N.; Malarz, J.; Kosecka-Strojek, M.; Musielak, B.; Stojakowska, A. Thymol derivatives from the roots of Xerolekia speciosissima an endemic species of the pre-Alpine area. Phytochem. Lett. 2019, 30, 235–237. [Google Scholar] [CrossRef]
- Talavera-Alemán, A.; Rodríguez-García, G.; López, Y.; García-Gutiérrez, H.A.; Torres-Valencia, J.; Del Río, R.E.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochem. Rev. 2015, 15, 251–277. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Willems, J.L.; Khamis, M.M.; Saeid, W.M.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, S.; Seger, C.; Wiesbauer, B.; Schneider, P.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpium Cass.) constituents. Phytochem. Anal. 2006, 17, 291–298. [Google Scholar] [CrossRef]
- Bazylko, A.; Boruc, K.; Borzym, J.; Kiss, A. Aqueous and ethanolic extracts of Galinsoga parviflora and Galinsoga ciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. Phytochem. Lett. 2015, 11, 394–398. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Kiss, A. Hydroxycinnamates from elecampane (Inula helenium L.) callus culture. Acta Physiol. Plant. 2016, 38, 41. [Google Scholar] [CrossRef] [Green Version]
- Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. Revisited: Newly Identified Constituents from Aerial Parts of the Plant and Their Possible Contribution to the Biological Activity of the Plant. Molecules 2019, 24, 1614. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, S.; Cervellati, R.; Seger, C.; Ellmerer, E.P.; About, N.; Renimel, I.; Godenir, C.; André, P.; Gafner, F.; Stuppner, H. Leontopodic acid—a novel highly substituted glucaric acid derivative from Edelweiss (Leontopodium alpinum Cass.) and its antioxidative and DNA protecting properties. Tetrahedron 2005, 61, 4621–4630. [Google Scholar] [CrossRef]
- Heilmann, J.; Müller, E.; Merfort, I. Flavonoid glucosides and dicaffeoylquinic acids from flowerheads of Buphthalmum salicifolium. Phytochemistry 1999, 51, 713–718. [Google Scholar] [CrossRef]
- Gohari, A.; Saeidnia, S.; Bayati-Moghadam, M.; Amin, G. Lignans and neolignans from Stelleropsis antoninae. DARU J. Pharm. Sci. 2011, 19, 74–79. [Google Scholar]
- Gutzeit, D.; Wray, V.; Winterhalter, P.; Jerz, G. Preparative isolation and purification of flavonoids and protocatechuic acid from sea buckthorn juice concentrate (Hippophaë rhamnoides L. ssp. rhamnoides) by high-speed counter-current chromatography. Chromatographia 2007, 65, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic characterization of Palatinate German white wines according to sensory attributes, varieties, and vintages using NMR spectroscopy and multivariate data analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Yuan, T.; Cirello, A.L.; Seeram, N.P. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012, 135, 1929–1937. [Google Scholar] [CrossRef]
- Kuczkowiak, U.; Petereit, F.; Nahrstedt, A. Hydroxycinnamic Acid Derivatives Obtained from a Commercial Crataegus Extract and from Authentic Crataegus spp. Sci. Pharm. 2014, 82, 835–846. [Google Scholar] [CrossRef] [Green Version]
- Merfort, I. Caffeoylquinic acids from flowers of Arnica montana and Arnica chamissonis. Phytochemistry 1992, 31, 2111–2113. [Google Scholar] [CrossRef]
- Lavoie, S.; Côté, I.; Pichette, A.; Gauthier, C.; Ouellet, M.; Nagau-Lavoie, F.; Mshvildadze, V.; Legault, J. Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis. BMC Complement. Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Shah, M.M. Acylated flavonol glycosides from Tagetes minuta with antibacterial activity. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and Anti-Inflammatory Activities of Quercetin 7-O-β-D-Glucopyranoside from the Leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef]
- Al-Attas, A.A.; El-Shaer, N.S.; Mohamed, G.A.; Ibrahim, S.R.; Esmat, A. Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes. J. Ethnopharmacol. 2015, 176, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Merfort, I.; Wendisch, D. Sesquiterpene lactones of Arnica cordifolia, subgenus Austromontana. Phytochemistry 1993, 34, 1436–1437. [Google Scholar] [CrossRef]
- Niu, J.; Huang, H.; Wang, F.; Zhang, X.; Liu, Y.; Yu, Q.; Hu, L. Synthetic derivatives of the natural product 13-amino 2-desoxy-4-epi-pulchellin inhibit STAT3 signaling and induce G2/M arrest and death of colon cancer cells. Bioorg. Med. Chem. Lett. 2019, 29, 782–785. [Google Scholar] [CrossRef]
- Ren, J.; Qin, J.J.; Cheng, X.R.; Yan, S.K.; Jin, H.-Z.; Zhang, W. Five new sesquiterpene lactones from Inula hupehensis. Arch. Pharmacal Res. 2013, 36, 1319–1325. [Google Scholar] [CrossRef]
- Rodrigues, A.A.S.; Garcia, M.; Rabi, J.A. Facile biomimetic synthesis of costunolide-1,10-epoxide, santamarin and reynosin. Phytochemistry 1978, 17, 953–954. [Google Scholar] [CrossRef]
- McDonald, P.P.; Bald, A.; Cassatella, M.A. Activation of the NF-κB Pathway by Inflammatory Stimuli in Human Neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts Beyond Expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Long-Term Cultured Hairy Roots of Chicory—A Rich Source of Hydroxycinnamates and 8-Deoxylactucin Glucoside. Appl. Biochem. Biotechnol. 2013, 171, 1589–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Żylewski, M.; Kisiel, W. Acylated hydroxycinnamic acid glucosides from flowers of Telekia Speciosa. Phytochem. Lett. 2015, 12, 257–261. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch.Bip. and Telekia speciosa (Schreb.) Baumg. (Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Tordera, M.; Ferrándiz, M.L.; Alcaraz, M.J. Influence of Anti-Inflammatory Flavonoids on Degranulation and Arachidonic Acid Release in Rat Neutrophils. Zeitschrift für Naturforschung C 1994, 49, 235–240. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-G.; Lee, D.-S.; Li, B.; Choi, Y.H.; Lee, S.-H.; Kim, Y.-C. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int. Immunopharmacol. 2012, 13, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-G.; Hong, X.; Li, L.; Zhou, J.; Hao, X.-J. Chemical Constituents of Two Chinese Magnoliaceae Plants, Tsoongiodendron odorum and Manglietiastrum sinicum, and their Inhibition of Platelet Aggregation. Planta Medica 2000, 66, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Ham, A.; Kim, D.-W.; Kim, K.H.; Lee, S.-J.; Oh, K.-B.; Shin, J.; Mar, W. Reynosin protects against neuronal toxicity in dopamine-induced SH-SY5Y cells and 6-hydroxydopamine-lesioned rats as models of Parkinson’s disease: Reciprocal up-regulation of E6-AP and down-regulation of α-synuclein. Brain Res. 2013, 1524, 54–61. [Google Scholar] [CrossRef]
- Wang, F.; Yang, K.; Ren, F.-C.; Liu, J.-K. Sesquiterpene lactones from Carpesium abrotanoides. Fitoterapia 2009, 80, 21–24. [Google Scholar] [CrossRef]
- Li, X.-W.; Weng, L.; Gao, X.; Zhao, Y.; Pang, F.; Liu, J.-H.; Zhang, H.; Hu, J.-F. Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi. Bioorganic Med. Chem. Lett. 2011, 21, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.-Y.; Qin, J.-J.; Huang, Y.; Yan, L.; Liu, Y.-B.; Pan, Y.-X.; Jin, H.-Z.; Zhang, W. Sesquiterpenoids from Inula lineariifolia Inhibit Nitric Oxide Production. J. Nat. Prod. 2010, 73, 1117–1120. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, Q.; Ren, J.; Qin, J.; Zhang, S.; Shen, Y.; Zhu, J.; Zhang, F.; Chang, R.; Zhu, Y.; et al. Sesquiterpene lactones from Inula falconeri, a plant endemic to the Himalayas, as potential anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 5408–5415. [Google Scholar] [CrossRef]
- Lee, H.-T.; Yang, S.-W.; Kim, K.H.; Seo, E.-K.; Mar, W. Pseudoguaianolides isolated from Inula britannica var. chinenis as inhibitory constituents against inducible nitric oxide synthase. Arch. Pharmacal Res. 2002, 25, 151–153. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F. Eremophilanolides, eudesmanolides, guaianolides and other constituents from Ondetia linearis. Phytochemistry 1989, 28, 1653–1660. [Google Scholar] [CrossRef]
- Bohlmann, F.; Mahanta, P.K. Zwei neue pseudoguajanolide aus Telekia speciosa. Phytochemistry 1979, 18, 887–888. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Polyacetylenverbindungen, 190. Notiz über die Inhaltsstoffe aus Buphthalmum salicifolium L. Eur. J. Inorg. Chem. 1971, 104, 958–960. [Google Scholar] [CrossRef]
- Stojakowska, A.; Galanty, A.; Malarz, J.; Michalik, M. Major terpenoids from Telekia speciosa flowers and their cytotoxic activity in vitro. Nat. Prod. Res. 2018, 33, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Wajs, A.; Stojakowska, A.; Kalemba, D. Chemical composition of essential oils from a multiple shoot culture of Telekia speciosa and different plant organs. Nat. Prod. Commun. 2012, 7, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; An, J.; Dong, Q.; Li, J.; Yang, W.; Sun, W.; Su, Z.; Zhang, S. Discovery of SCY45, a Natural Small-Molecule MDM2-p53 Interaction Inhibitor. Chem. Biodivers. 2019, 16, e1900081. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef]







| No | Compound | tR [min] | UV [nm] | [M − H]− | Product Ion Main Peaks 1 | R 2 | A 3 |
|---|---|---|---|---|---|---|---|
| 1 | 3-O-Caffeoylquinic acid (3-CQA) | 3.1 | 325 | 353 | 191, 179 | + | + |
| 2 | 5-O-Caffeoylquinic acid (5-CQA) | 5.1 | 325 | 353 | 191 | + | + |
| 3 | 4-O-Caffeoyl quinic acid (4-CQA) | 5.9 | 325 | 353 | 191, 179, 173 | + | − |
| 4 | Dicaffeoyl hexaric acid | 7.9 | 324 | 533 | 371, 209 | + | + |
| 5 | 1,3-Di-O-caffeoyl quinic acid (1,3-DCQA) | 8.4 | 322 | 515 | 353, 335, 191, 179 | + | − |
| 6 | Quercetin hexoside | 11.3 | 274, 343 | 463 | 301 | − | + |
| 7 | Kaempferol rhamnosylhexoside | 12.3 | 265, 341 | 593 | 285 | − | + |
| 8 | 3,4-Di-O-caffeoyl quinic acid (3,4-DCQA) | 12.5 | 325 | 515 | 353, 335, 299, 255, 203, 191, 179, 173 | + | + |
| 9 | 1,5-Di-O-caffeoyl quinic acid (1,5-DCQA) | 12.9 | 327 | 515 | 353, 191 | + | + |
| 10 | Tricaffeoyl hexaric acid (I) | 13.5 | 327 | 695 | 533, 371, 209 | + | − |
| 11 | 4,5-Di-O-caffeoyl quinic acid (4,5-DCQA) | 13.8 | 327 | 515 | 353, 317, 299, 255, 203, 173 | + | + |
| 12 | Dicaffeoyl quinic acid derivative | 14.4 | 326 | 601 | 439, 353, 335, 179 | − | + |
| 13 | Tricaffeoyl hexaric acid (II) | 14.8 | 328 | 695 | 533, 371, 209 | − | + |
| 14 | Tricaffeoyl hexaric acid (III) | 15.0 | 328 | 695 | 533, 371, 209 | − | + |
| 15 | Hydroxybutyryl-tricaffeoyl hexaric acid (leontopodic acid) | 16.1 | 327 | 781 | 619, 457, 295 | + | − |
| 16 | Tetracaffeoyl hexaric acid | 19.5 | 328 | 857 | 698, 533, 371, 209 | + | − |
| 17 | Isobutyryl-dicaffeoyl quinic acid | 19.8 | 328 | 585 | 497, 423, 335, 179 | − | + |
| 18 | Methylbutyryl/isovaleryl-dicaffeoyl quinic acid | 23.7 | 328 | 599 | 497, 437, 335, 179 | − | + |
| 19 | Isobutyryl-tricaffeoyl hexaric acid | 24.6 | 328 | 765 | 603, 441, 279 | + | − |
| Compound | tR [min] | Content in Leaves [% Dry Weight] 1 | ||
|---|---|---|---|---|
| X. speciosissima | B. salicifolium | T. speciosa | ||
| 3-CQA (1) | 5.2 | 0.029 ± 0.010 | 0.165 ± 0.097 | 0.016 ± 0.004 |
| 5-CQA (2) | 7.0 | 0.654 ± 0.236 | 0.815 ± 0.330 | 1.063 ± 0.081 |
| 4-CQA (3) | 8.0 | 0.130 ± 0.046 | 0.033 ± 0.012 | 0.032 ± 0.003 |
| Dicaffeoylhexaric acid (4) | 8.8 | 0.486 ± 0.142 | - | - |
| 3,4-DCQA (8) | 22.6 | 0.718 ± 0.242 | 0.909 ± 0.262 | 0.561 ± 0.146 |
| 1,5-DCQA (9) | 23.4 | 1.286 ± 0.342 | 0.536 ± 0.194 | 0.350 ± 0.085 |
| 3,5-DCQA | 24.9 | - | - | 0.232 ± 0.032 |
| 4,5-DCQA (11) | 31.2 | 0.029 ± 0.007 | 0.060 ± 0.010 | 0.044 ± 0.017 |
| Dicaffeoylquinic acid derivative (12) | 36.6 | 0.020 ± 0.004 | - | - |
| Tricaffeoylhexaric acid (II) (13) | 39.2 | 0.403 ± 0.230 | - | - |
| Isobutyryl-dicaffeoylquinic acid (17) | 55.3 | 0.258 ± 0.101 | 0.724 ± 0.380 | - |
| Methylbutyryl/isovaleryl-dicaffeoylquinic acid (18) | 58.6 | 0.097 ± 0.043 | 0.037 ± 0.013 | - |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłeczek, N.; Malarz, J.; Gierlikowska, B.; Kiss, A.K.; Stojakowska, A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate. Molecules 2020, 25, 4913. https://doi.org/10.3390/molecules25214913
Kłeczek N, Malarz J, Gierlikowska B, Kiss AK, Stojakowska A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate. Molecules. 2020; 25(21):4913. https://doi.org/10.3390/molecules25214913
Chicago/Turabian StyleKłeczek, Natalia, Janusz Malarz, Barbara Gierlikowska, Anna K. Kiss, and Anna Stojakowska. 2020. "Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate" Molecules 25, no. 21: 4913. https://doi.org/10.3390/molecules25214913