Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals
Abstract
:1. Introduction
2. Results and Discussion
2.1. FT-IR Spectroscopic Confirmation of SMHB Complexes Formation
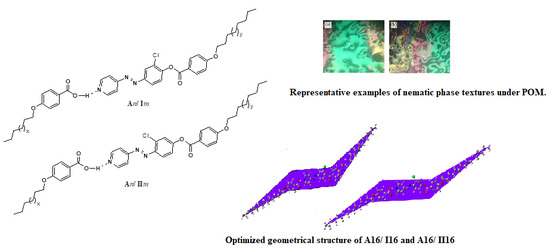
2.2. Mesomorphic and Optical Behavior
2.3. Effect of Polarity and Orientation of Lateral Substituent on the Supramolecular Hydrogen-Bonded Complexes Stability
2.4. DFT Calculations
2.4.1. Relationship between Experimental and Theoretical Parameters
2.4.2. Entropy Change of SMHB Complexes
2.4.3. Frontier Molecular Orbitals and Molecular Electrostatic Potential
3. Experimental
3.1. Preparation of 1:1 Supramolecular Complexes
3.2. Characterizations
3.3. Computational Methods and Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shen, P.; Zhang, X.; Lu, H.; Su, Z.; Zhou, Y.; Song, B.; Li, X.; Yang, X.; Tu, Y.; Li, C.Y. Effect of Fullerene Volume Fraction on Two-Dimensional Crystal-Constructed Supramolecular Liquid Crystals. Chem. Asian J. 2019, 14, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Dechant, M.; Gerbig, L.; Baumann, M. Supramolecular click procedures in liquid crystals. Liq. Cryst. 2019, 46, 1–10. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Kather, S.; Wölper, C.; Daniliuc, C.; Mezger, M.; Giese, M. Improving the mesomorphic behaviour of supramolecular liquid crystals by resonance-assisted hydrogen bonding. J. Mater. Chem. C 2019. [Google Scholar] [CrossRef]
- Sharma, V.S.; Shah, A.P.; Sharma, A.S. A new class of supramolecular liquid crystals derived from azo calix [4] arene functionalized 1, 3, 4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Kong, S.; Song, Y.; Meng, F. Star-shaped supramolecular ionic liquid crystals based on pyridinium salts. Liq. Cryst. 2019, 46, 512–522. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Frechet, J.M. Supramolecular liquid-crystalline networks built by self-assembly of multifunctional hydrogen-bonding molecules. Chem. Mater. 1996, 8, 961–968. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Frechet, J.M. Induction of a cholesteric phase via self-assembly in supramolecular networks built of non-mesomorphic molecular components. Liq. Cryst. 1998, 24, 413–418. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4′′-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Gowda, A.; Jacob, L.; Joy, N.; Philip, R.; Pratibha, R.; Kumar, S. Thermal and nonlinear optical studies of newly synthesized EDOT based bent-core and hockey-stick like liquid crystals. New J. Chem. 2018, 42, 2047–2057. [Google Scholar] [CrossRef]
- Gray, G.W.; Jones, B. The Mesomorphic Transition Points of the Para-Normal-Alkoxybenzoic Acids-A Correction; Royal Society of Chemistry: Cambridge, UK, 1953; pp. 4179–4180. [Google Scholar]
- Kato, T.; Frechet, J.M. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Kato, T.; Wilson, P.G.; Fujishima, A.; Fréchet, J.M. Hydrogen-bonded liquid crystals. A novel mesogen incorporating nonmesogenic 4,4′-bipyridine through selective recognition between hydrogen bonding donor and acceptor. Chem. Lett. 1990, 19, 2003–2006. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.; Wilson, P.G.; Saito, T.; Uryu, T.; Fujishima, A.; Jin, C.; Kaneuchi, F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenic bipyridyl compounds through complexation between hydrogen-bond donor and acceptor moieties. Chem. Mater. 1993, 5, 1094–1100. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M. Hydrogen Bonding and the Self-Assembly of Supramolecular Liquid-Crystalline Materials; Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 1995; pp. 311–326. [Google Scholar]
- Yagai, S.; Kitamura, A. Recent advances in photoresponsive supramolecular self-assemblies. Chem. Soc. Rev. 2008, 37, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Jiao, D.; Biedermann, F.; Scherman, O.A. Orthogonal switching of a single supramolecular complex. Nat. Commun. 2012, 3, 1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Grubert, L.; Hecht, S.; Bléger, D. Orthogonal switching in four-state azobenzene mixed-dimers. Chem. Commun. 2017, 53, 3323–3326. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Quan, M.; Zhang, L.; Yang, H.; Lu, Y. Photothermal effect of azopyridine compounds and their applications. RSC Adv. 2015, 5, 4675–4680. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Cuadrado, A.; Ortega, M.; Nonell, S.; Velasco, D. A photoswitchable bis-azo derivative with a high temporal resolution. Chem. Commun. 2014, 50, 11462–11464. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff Base/Ester Liquid Crystals with Different Lateral Substituents: Mesophase Behaviour and DFT Calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Abd Eltawab, M.M.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Ahmed, H.; Saad, G. Mesophase behaviour of laterally di-fluoro-substituted four-ring compounds. Liq. Cryst. 2015, 42, 1765–1772. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Effect of the relative orientation of the two fluoro-substituents on the mesophase behavior of phenylazophenyl benzoates. Mol. Cryst. Liq. Cryst. 2012, 562, 43–65. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Liquid crystalline behaviour of model compounds di-laterally substituted with different polar groups. Liq. Cryst. 2011, 38, 511–519. [Google Scholar] [CrossRef]
- Naoum, M.; Ahmed, H. Effect of dipole moment and conformation on the mesophase behavior of di-laterally substituted phenylazophenyl benzoate liquid crystals. Thermochim. Acta 2011, 521, 202–210. [Google Scholar] [CrossRef]
- Naoum, M.; Mohammady, S.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-) methyl phenyl-4’-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, H. Different morphologies of self-assembled nanofibers fabricated with amphiphilic low-molecular-weight azopyridinium salts. RSC Adv. 2013, 3, 22155–22159. [Google Scholar] [CrossRef]
- Zhou, W.; Kobayashi, T.; Zhu, H.; Yu, H. Electrically conductive hybrid nanofibers constructed with two amphiphilic salt components. Chem. Commun. 2011, 47, 12768–12770. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, R.; Jackson, J.K.; Chiao, M.; Yu, H. Janus ultrathin film from multi-level self-assembly at air–water interfaces. Chem. Commun. 2014, 50, 14843–14846. [Google Scholar] [CrossRef]
- Mamiya, J.-I.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65. [Google Scholar] [CrossRef]
- Aoki, K.I.; Nakagawa, M.; Ichimura, K. Self-assembly of amphoteric azopyridine carboxylic acids: Organized structures and macroscopic organized morphology influenced by heat, pH change, and light. J. Am. Chem. Soc. 2000, 122, 10997–11004. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M.A. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular hydrogen-bonded liquid crystals formed from 4-(4′-pyridylazophenyl)-4″-alkoxy benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2008, 487, 74–91. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular liquid crystals induced by hydrogen-bonding interactions between non-mesomorphic compounds. I. 4-(4′-Pyridylazophenyl)-4″-substituted benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2009, 506, 22–33. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.G.A.; Almllal, W.A. Supramolecular Liquid Crystals Induced by Hydrogen-Bonding Interactions between Non-Mesomorphic Compounds. II. Effect of Lateral Substitution. Mol. Cryst. Liq. Cryst. 2010, 518, 109–128. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Chen, K.-Y. Crystal Structure, Hydrogen-Bonding Properties, and DFT Studies of 2-((2-(2-Hydroxyphenyl) benzo [d] thiazol-6-yl) methylene) malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Shoji, M.; Tanaka, F. Theoretical study of hydrogen-bonded supramolecular liquid crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and Mesophase Study of Coumarin Esters and Its Azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Quinazolin-4-yl-sulfanylacetyl-hydrazone derivatives; Synthesis, molecular structure and electronic properties. J. Mol. Struct. 2013, 1049, 177–188. [Google Scholar] [CrossRef]
- Soliman, S.M.; Hagar, M.; Ibid, F.; El Sayed, H. Experimental and theoretical spectroscopic studies, HOMO–LUMO, NBO analyses and thione–thiol tautomerism of a new hybrid of 1, 3, 4-oxadiazole-thione with quinazolin-4-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4 (3H)-one derivatives; an account on the N-versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Hagar, M.; Soliman, S.M. Ultrasonic Synthesis, Molecular Structure and Mechanistic Study of 1, 3-Dipolar Cycloaddition Reaction of 1-Alkynylpyridinium-3-olate and Acetylene Derivatives. Molecules 2016, 21, 848. [Google Scholar] [CrossRef] [Green Version]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernández, B.; Diez-Berart, S.; Salud, J.; de la Fuente, M.R.; Timimi, B.A. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-4′-yloxy)-6-(4-cyanobiphenyl-4′-yl) hexane (CB6OCB) and comparison with CB7CB. Soft Matter 2016, 12, 6827–6840. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Ahmed, H.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino) phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Lateral substituent effects on UV stability of high-birefringence liquid crystals with the diaryl-diacetylene core: DFT/TD-DFT study. Liq. Cryst. 2017, 44, 1515–1524. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Parveen, S.; Hagar, M.; Ahmed, H.A.; Knight, J.G. A New Chiral Boron–dipyrromethene (BODIPY)–based Fluorescent Probe: Molecular docking, DFT, Antibacterial and Antioxidant approaches. J. Biomol. Struct. Dyn. 2019. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2019. [Google Scholar] [CrossRef]
- Alnoman, R.; Ahmed, H.A.; Hagar, M. Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Chaieb, K.; Parveen, S.; Ahmed, H.; Alnoman, R. N-alkyl 2-pyridone versus O-alkyl 2-pyridol: Ultrasonic synthesis, DFT, docking studies and their antimicrobial evaluation. J. Mol. Struct. 2020, 1199, 126926. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef] [Green Version]
- Cleland, W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Lizu, M.; Lutfor, M.; Surugau, N.; How, S.; Arshad, S.E. Synthesis and characterization of ethyl cellulose–based liquid crystals containing azobenzene chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noel, C. FTIR investigation of two alkyl-p-terphenyl-4, 4 ″-dicarboxylates in their crystalline, smectic and isotropic phases. Mol. Cryst. Liq. Cryst. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.M.; Storey, J.M.D.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.; Martinez-Felipe, A.; Paterson, D.; Forsyth, E.; Lawrence, G.; Henderson, P.; Storey, J.; Gorecka, E. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1:1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Thaker, B.; Kanojiya, J.; Tandel, R. Effects of different terminal substituents on the mesomorphic behavior of some azo-schiff base and azo-ester-based liquid crystals. Mol. Cryst. Liq. Cryst. 2010, 528, 120–137. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A.; Salem, R.A. Supramolecular liquid crystals in binary and ternary systems. Thermochim. Acta 2011, 517, 63–73. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Saad, G. Impact of the proportionation of dialkoxy chain length on the mesophase behaviour of Schiff base/ester liquid crystals; experimental and theoretical study. Liq. Cryst. 2019, 46, 1–10. [Google Scholar] [CrossRef]
- Imrie, C. Laterally substituted dimeric liquid crystals. Liq. Cryst. 1989, 6, 391–396. [Google Scholar] [CrossRef]
- Schroeder, J.; Bristol, D. Liquid crystals. IV. Effects of terminal substituents on the nematic mesomorphism of p-phenylene dibenzoates. J. Org. Chem. 1973, 38, 3160–3164. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1–12. [Google Scholar] [CrossRef]
- Cammenga, H.K.; Eysel, W.; Gmelin, E.; Hemminger, W.; Höhne, G.W.; Sarge, S.M. The temperature calibration of scanning calorimeters: Part 2. Calibration substances. Thermochim. Acta 1993, 219, 333–342. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A 02; Gaussian Inc.: Wallingford, CT, USA, 2009; p. 200. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
Sample Availability: Samples of all compounds are available from the authors. |














| Compound | ύOH (cm−1) | O-H (Å) | ύC=O (cm−1) | C=O (Å) | ύC=NPyr (cm−1) | C=NPyr (Å) | ύH-bond (cm−1) | H-Bond (Å) |
|---|---|---|---|---|---|---|---|---|
| A12 | 3660.9 | 0.976 | 1691.0 | 1.237 | ||||
| I16 | 1595 | 1.356 | ||||||
| II16 | 1593.5 | 1.351 | ||||||
| A12/I16 | 2511.4 | 1.040 | 1686.8 | 1.255 | 1609.2 | 1.354 | 2511.4 | 1.606 |
| A12/II16 | 2573.0 | 1.032 | 1666.6 | 1.252 | 1599.4 | 1.352 | 2573.0 | 1.620 |
| System | TCr-N | ∆HCr-N | TN-I | ∆HN-I | ∆S/RN-I |
|---|---|---|---|---|---|
| A6/I8 | 60.1 | 71.75 | 77.3 | 1.66 | 0.57 |
| A8/I8 | 61.9 | 61.59 | 75.8 | 1.56 | 0.54 |
| A10/I8 | 66.2 | 79.71 | 80.0 | 2.45 | 0.83 |
| A12/I8 | 68.2 | 68.49 | 80.1 | 2.32 | 0.79 |
| A6/I16 | 74.4 | 87.51 | 89.8 | 2.32 | 0.77 |
| A8/I16 | 73.7 | 89.04 | 87.1 | 1.96 | 0.65 |
| A10/I16 | 77.8 | 87.68 | 86.3 | 3.36 | 1.12 |
| A12/I16 | 80.5 | 98.32 | 71.5 * | 2.76 | 0.96 |
| A6/II8 | 77.9 | 83.41 | 114.3 | 2.85 | 0.88 |
| A8/II8 | 77.3 | 80.56 | 87.4 | 1.96 | 0.65 |
| A10/II8 | 81.8 | 84.80 | 79.5 * | 2.08 | 0.71 |
| A12/II8 | 84.9 | 86.50 | 50.1 * | 2.97 | 1.11 |
| A6/II16 | 88.1 | 92.56 | 82.9 * | 3.16 | 1.07 |
| A8/II16 | 90.1 | 94.13 | 82.5 * | 2.60 | 0.88 |
| A10/II16 | 92.2 | 96.16 | 79.0 * | 3.50 | 1.20 |
| A12/II16 | 93.3 | 96.34 | 80.4 * | 3.63 | 1.23 |
| Parameter | A12/I16 | A16/I16 | A6/II16 | A8/II16 | A10/II16 | A12/II16 | A16/II16 |
|---|---|---|---|---|---|---|---|
| Ecorr | 1.187 | 1.301 | 1.023 | 1.081 | 1.139 | 1.196 | 1.311 |
| ZPVE | −3138.763 | −3295.897 | −2902.467 | −2981.018 | −3059.569 | −3138.119 | −3295.220 |
| Etot | −3138.694 | −3295.822 | −2902.407 | −2980.954 | −3059.502 | −3138.050 | −3295.146 |
| H | −3138.693 | −3295.821 | −2902.406 | −2980.954 | −3059.501 | −3138.049 | −3295.145 |
| G | −3138.893 | −3296.035 | −2902.584 | −2981.138 | −3059.692 | −3138.246 | −3295.356 |
| Total Dipole Moment | 6.840 | 6.841 | 8.889 | 8.881 | 8.865 | 8.863 | 8.860 |
| Polarizability α | 741.05 | 788.27 | 638.07 | 660.73 | 683.18 | 705.30 | 749.78 |
| Compound | EHOMO (a.u) | ELUMO (a.u) | ΔE(ELUMO–EHOMO) (a.u) | S = 1/ΔE |
|---|---|---|---|---|
| A12/I16 | −0.215 | −0.114 | 0.100 | 9.959 |
| A16/I16 | −0.225 | −0.123 | 0.102 | 9.766 |
| A6/II16 | −0.215 | −0.115 | 0.100 | 9.969 |
| A8/II16 | −0.215 | −0.114 | 0.100 | 9.965 |
| A10/II16 | −0.215 | −0.114 | 0.100 | 9.962 |
| A12/II16 | −0.215 | −0.114 | 0.100 | 9.959 |
| A16/II16 | −0.215 | −0.114 | 0.100 | 9.958 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaddad, O.A.; Ahmed, H.A.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. https://doi.org/10.3390/molecules25020365
Alhaddad OA, Ahmed HA, Hagar M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules. 2020; 25(2):365. https://doi.org/10.3390/molecules25020365
Chicago/Turabian StyleAlhaddad, O. A., H. A. Ahmed, and M. Hagar. 2020. "Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals" Molecules 25, no. 2: 365. https://doi.org/10.3390/molecules25020365