3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Studies
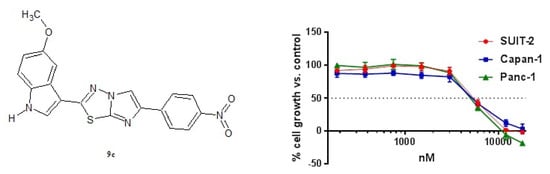
2.2.1. Antiproliferative Activity of the New Imidazothiadiazole Compounds 9a–p on SUIT-2, Capan-1 and Panc-1 Pancreatic Cancer Cells
2.2.2. Compound 9c Inhibited the Migration Rate in SUIT-2, Capan-1 and Panc-1 Cells
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 1H-indole-3-carbonitriles (5a,b)
3.1.2. Synthesis of 1-methylindole-3-carbonitriles (6a,b)
3.1.3. Synthesis of 5-(1H-indol-3-yl)-1,3,4-thiadiazol-2-amines (7a–d)
5-(5-Methoxy-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7a)
5-(5-Methoxy-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7b)
5-(5-Fluoro-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7c)
5-(5-Fluoro-1-methyl-1H-indol-3-yl)-1,3,4-thiadiazol-2-amine (7d)
3.1.4. Synthesis of 3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromides (9a–p)
5-Methoxy-3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromide 9a
5-Methoxy-3-[6-(4-fluorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1H-indole hydrobromide 9b
5-Methoxy-3-[6-(4-nitrophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole 9c
5-Methoxy-3-[6-(3-methoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9d
5-Methoxy-3-[6-(2,5-dimethoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1H-indole 9e
5-Methoxy-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole hydrobromide 9f
5-Methoxy-1-methyl-3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromide 9g
5-Methoxy-3-[6-(4-fluorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1-methyl-1H-indole hydrobromide 9h
5-Methoxy-1-methyl-3-[6-(3-methoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9i
5-Methoxy-3-[6-(2,5-dimethoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-5-methoxy-1-methyl-1H-indole 9j
5-Methoxy-1-methyl-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole 9k
5-Fluoro-3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole hydrobromide 9l
5-Fluoro-3-[6-(3-methoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9m
5-Fluoro-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole hydrobromide 9n
5-Fluoro-3-[6-(2,5-dimethoxyphenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl]-1H-indole hydrobromide 9o
5-Fluoro-1-methyl-3-{6-[4-(trifluoromethyl)phenyl]imidazo[2,1-b][1,3,4]thiadiazol-2-yl}-1H-indole hydrobromide 9p
3.2. Biology
3.2.1. Drugs and Chemical
3.2.2. Cell Cultures
3.2.3. Cell Growth Inhibition
3.2.4. Wound-Healing Assays
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, V.B.; Kulkarni, M.V.; Rasal, V.P.; Biradar, S.S.; Vinay, M.D. Synthesis and anti-inflammatory evaluation of methylene bridged benzofuranyl imidazo[2,1-b][1,3,4] thiadiazoles. Eur. J. Med. Chem. 2008, 43, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, A.; Razmi, S.; Mahdavi, M.; Foroumadi, P.; Ardestani, S.K.; Emami, S.; Kobarfard, F.; Dastmalchi, S.; Shafiee, A.; Foroumadi, A. Synthesis and anti-leishmanial activity of 5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-amines containing N-[(1-benzyl-1H-1,2,3-triazol-4-yl) methyl] moieties. Eur. J. Med. Chem. 2012, 50, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, K.; Matić, I.Z.; Stanojković, T.; Krivokuća, A.; Marković, V.; Joksović, M.D.; Mihailović, N.; Nićiforović, M.; Joksović, L. Synthesis, antioxidant and antiproliferative activities of 1,3,4-thiadiazoles derived from phenolic acids. Bioorg. Med. Chem. Lett. 2017, 27, 3709–3715. [Google Scholar] [CrossRef]
- Alegaon, S.G.; Alagawadi, K.R.; Sonkusare, P.V.; Chaudhary, S.M.; Dadwe, D.H.; Shah, A.S. Novel imidazo[2,1-b] [1,3,4] thiadiazole carrying rhodanine-3-acetic acid as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2012, 22, 1917–1921. [Google Scholar] [CrossRef]
- Bhongade, B.A.; Talath, S.; Gadad, R.A.; Gadad, A.K. Biological activities of imidazo[2,1-b] [1,3,4] thiadiazole derivatives: A review. J. Saudi Chem. Soc. 2016, 20, S463–S475. [Google Scholar] [CrossRef] [Green Version]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Petri, G.L.; Cusimano, M.G.; Schillaci, D.; Di Sarno, V.; Musella, S.; Giovannetti, E.; Cirrincione, G.; Diana, P. 2,6-Disubstituted imidazo[2,1-b] [1,3,4] thiadiazole derivatives as potent staphylococcal biofilm inhibitors. Eur. J. Med. Chem. 2019, 167, 200–210. [Google Scholar] [CrossRef]
- Parrino, B.; Schillaci, D.; Carnevale, I.; Giovannetti, E.; Diana, P.; Cirrincione, G.; Cascioferro, S. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur. J. Med. Chem. 2019, 161, 154–178. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.S.; Panjamurthy, K.; Kumar, S.; Nambiar, M.; Ramareddy, S.A.; Chiruvella, K.K.; Raghavan, S.C. Synthesis and biological evaluation of novel 2-aralkyl-5-substituted-6-(4′-fluorophenyl)-imidazo[2,1-b] [1,3,4] thiadiazole derivatives as potent anticancer agents. Eur. J. Med. Chem. 2011, 46, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hegde, M.; Gopalakrishnan, V.; Renuka, V.K.; Ramareddy, S.A.; De Clercq, E.; Schols, D.; Gudibabande Narasimhamurthy, A.K.; Raghavan, S.C.; Karki, S.S. 2-(4-Chlorobenzyl)-6-arylimidazo[2,1-b] [1,3,4] thiadiazoles: Synthesis, cytotoxic activity and mechanism of action. Eur. J. Med. Chem. 2014, 84, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, O.K.; Hussein, W.M.; Vella, P.; Yusof, Y.; Sidjabat, H.E.; Schenk, G.; McGeary, R.P. Design, synthesis, and in vitro and biological evaluation of potent amino acid-derived thiol inhibitors of the metallo-β-lactamase IMP-1. Eur. J. Med. Chem. 2016, 114, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romagnoli, R.; Baraldi, P.G.; Prencipe, F.; Balzarini, J.; Liekens, S.; Estévez, F. Design, synthesis and antiproliferative activity of novel heterobivalent hybrids based on imidazo[2,1-b] [1,3,4] thiadiazole and imidazo[2,1-b] [1,3] thiazole scaffolds. Eur. J. Med. Chem. 2015, 101, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gopalakrishnan, V.; Hegde, M.; Rana, V.; Dhepe, S.S.; Ramareddy, S.A.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; et al. Synthesis and antiproliferative activity of imidazo[2,1-b] [1,3,4] thiadiazole derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 4682–4688. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.M.; Sing, B.; Bhardwaj, V.; Palkar, M.; Shaikh, M.S.; Rane, R.; Alwan, W.S.; Gadad, A.K.; Noolvi, M.N.; Karpoormath, R. Design, synthesis and evaluation of small molecule imidazo[2,1-b] [1,3,4] thiadiazoles as inhibitors of transforming growth factor-β type-I receptor kinase (ALK5). Eur. J. Med. Chem. 2015, 93, 599–613. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a] quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef]
- Parrino, B.; Ullo, S.; Attanzio, A.; Cascioferro, S.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Tesoriere, L.; et al. Synthesis of 5H-pyrido[3,2-b] pyrrolizin-5-one tripentone analogs with antitumor activity. Eur. J. Med. Chem. 2018, 158, 236–246. [Google Scholar] [CrossRef]
- Diana, P.; Stagno, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of the new ring system pyrrolizino[2,3-b] indol-4(5H)-one. Tetrahedron 2011, 67, 3374–3379. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, A.; Spanò, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Sissi, C.; Palumbo, M.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Stagno, A.; Barraja, P.; Carbone, A.; Parrino, B.; Dall’Acqua, F.; Vedaldi, D.; Salvador, A.; Brun, P.; Castagliuolo, I.; et al. Synthesis of triazenoazaindoles: A new class of triazenes with antitumor activity. ChemMedChem 2011, 6, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Ciancimino, C.; Carbone, A.; Spano’, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P. Synthesis of isoindolo [1,4] benzoxazinone and isoindolo [1,5] benzoxazepine: Two new ring systems of pharmaceutical interest. Tetrahedron 2015, 71, 7332–7338. [Google Scholar] [CrossRef]
- Cascioferro, S.; Attanzio, A.; Di Sarno, V.; Musella, S.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Mar. Drugs 2019, 17, 35. [Google Scholar] [CrossRef] [Green Version]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef]
- Spanò, V.; Attanzio, A.; Cascioferro, S.; Carbone, A.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. Synthesis and Antitumor Activity of New Thiazole Nortopsentin Analogs. Mar. Drugs 2016, 14, 226. [Google Scholar] [CrossRef] [Green Version]
- Carbone, A.; Parrino, B.; Di Vita, G.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; Diana, P.; et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo [2,3-b] pyridines and indolyl-thiazolyl-pyrrolo [2,3-c] pyridines, nortopsentin analogues. Mar. Drugs 2015, 13, 460–492. [Google Scholar] [CrossRef] [Green Version]
- Parrino, B.; Carbone, A.; Di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; et al. 3-[4-(1H-indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b] pyridines, nortopsentin analogues with antiproliferative activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef] [Green Version]
- Meijer, L.L.; Garajova, I.; Caparello, C.; Le Large, T.Y.; Frampton, A.; Vasile, E.; Fune, N.; Kazemier, G.; Giovannetti, E. Plasma miR-181a-5p Down-Regulation Predicts Response and Improved Survival After FOLFIRINOX in Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- El Hassouni, B.; Li Petri, G.; Liu, D.; Cascioferro, S.; Parrino, B.; Hassan, W.; Diana, P.; Ali, A.; Frampton, A.E.; Giovannetti, E. Pharmacogenetics of treatments for pancreatic cancer. Expert Opin. Drug Metab. Toxicol. 2019, 15, 437–447. [Google Scholar] [CrossRef]
- Le Large, T.Y.S.; El Hassouni, B.; Funel, N.; Kok, B.; Piersma, S.R.; Pham, T.V.; Olive, K.P.; Kazemier, G.; van Laarhoven, H.W.M.; Jimenez, C.R.; et al. Proteomic analysis of gemcitabine-resistant pancreatic cancer cells reveals that microtubule-associated protein 2 upregulation associates with taxane treatment. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, E.; van der Borden, C.L.; Frampton, A.E.; Ali, A.; Firuzi, O.; Peters, G.J. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin. Cancer Biol. 2017, 44, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Le Large, T.Y.S.; Bijlsma, M.F.; Kazemier, G.; van Laarhoven, H.W.M.; Giovannetti, E.; Jimenez, C.R. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin. Cancer Biol. 2017, 44, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, T.; Caffrey, T.C.; Kitamura, N.; Yamanari, H.; Setoguchi, T.; Hollingsworth, M.A. P-selectin expression in a metastatic pancreatic tumor cell line (SUIT-2). Cancer Res. 1997, 57, 1206–1212. [Google Scholar] [PubMed]
- Deer, E.L.; Gonzalez-Hernandez, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maftouh, M.; Avan, A.; Funel, N.; Frampton, A.E.; Fiuji, H.; Pelliccioni, S.; Castellano, L.; Galla, V.; Peters, G.J.; Giovannetti, E. miR-211 modulates gemcitabine activity through downregulation of ribonucleotide reductase and inhibits the invasive behavior of pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids 2014, 33, 384–393. [Google Scholar] [CrossRef]
- Avan, A.; Crea, F.; Paolicchi, E.; Funel, N.; Galvani, E.; Marquez, V.E.; Honeywell, R.J.; Danesi, R.; Peters, G.J.; Giovannetti, E. Molecular Mechanisms Involved in the Synergistic Interaction of the EZH2 Inhibitor 3-Deazaneplanocin A with Gemcitabine in Pancreatic Cancer Cells. Mol. Cancer Ther. 2012, 11, 1735–1746. [Google Scholar] [CrossRef] [Green Version]
- Avan, A.; Caretti, V.; Funel, N.; Galvani, E.; Maftouh, M.; Honeywell, R.J.; Lagerweij, T.; Van Tellingen, O.; Campani, D.; Fuchs, D.; et al. Crizotinib inhibits metabolic inactivation of gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res. 2013, 73, 6745–6756. [Google Scholar] [CrossRef] [Green Version]
- Ebos, J.M.L.; Lee, C.R.; Cruz-Munoz, W.; Bjarnason, G.A.; Christensen, J.G.; Kerbel, R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009, 15, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Carbone, A.; Parrino, B.; Cusimano, M.G.; Spanò, V.; Montalbano, A.; Barraja, P.; Schillaci, D.; Cirrincione, G.; Diana, P.; Cascioferro, S. New Thiazole Nortopsentin Analogues Inhibit Bacterial Biofilm Formation. Mar. Drugs 2018, 16, 274. [Google Scholar] [CrossRef] [Green Version]
- Boosa, V.; Bilakanti, V.; Velisoju, V.K.; Gutta, N.; Inkollu, S.; Akula, V. An insight on the influence of surface Lewis acid sites for regioselective CH bond C3-cyanation of indole using NH4I and DMF as combined cyanide source over Cu/SBA-15 catalyst. Mol. Catal. 2018, 445, 43–51. [Google Scholar] [CrossRef]
- Sciarrillo, R.; Wojtuszkiewicz, A.; Kooi, I.E.; Gómez, V.E.; Boggi, U.; Jansen, G.; Kaspers, G.-J.; Cloos, J.; Giovannetti, E. Using RNA-sequencing to Detect Novel Splice Variants Related to Drug Resistance in In Vitro Cancer Models. J. Vis. Exp. 2016, 118, 54714. [Google Scholar] [CrossRef] [PubMed]
- Massihnia, D.; Avan, A.; Funel, N.; Maftouh, M.; van Krieken, A.; Granchi, C.; Raktoe, R.; Boggi, U.; Aicher, B.; Minutolo, F.; et al. Phospho-Akt overexpression is prognostic and can be used to tailor the synergistic interaction of Akt inhibitors with gemcitabine in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Caparello, C.; Meijer, L.L.; Garajova, I.; Falcone, A.; Le Large, T.Y.; Funel, N.; Kazemier, G.; Peters, G.J.; Vasile, E.; Giovannetti, E. FOLFIRINOX and translational studies: Towards personalized therapy in pancreatic cancer. World J. Gastroenterol 2016, 22, 6987–7005. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Compound | R | R1 | R2 | Yield (%) |
|---|---|---|---|---|
| 9a | OCH3 | H | H | 80% |
| 9b | OCH3 | H | 4-F | 75% |
| 9c | OCH3 | H | 4-NO2 | 73% |
| 9d | OCH3 | H | 3-OCH3 | 78% |
| 9e | OCH3 | H | 2,5-OCH3 | 78% |
| 9f | OCH3 | H | 4-CF3 | 68% |
| 9g | OCH3 | CH3 | H | 81% |
| 9h | OCH3 | CH3 | 4-F | 72% |
| 9i | OCH3 | CH3 | 3-OCH3 | 60% |
| 9j | OCH3 | CH3 | 2,5-OCH3 | 65% |
| 9k | OCH3 | CH3 | 4-CF3 | 67% |
| 9l | F | H | H | 72% |
| 9m | F | H | 3-OCH3 | 74% |
| 9n | F | H | 4-CF3 | 68% |
| 9o | F | H | 2,5-OCH3 | 76% |
| 9p | F | CH3 | 4-CF3 | 78% |
| IC50 a (µM) ± SEM b Cell Lines | |||
|---|---|---|---|
| Comp | SUIT-2 | Capan-1 | Panc-1 |
| 9a | >16 | >16 | >16 |
| 9b | >16 | >16 | >16 |
| 9c | 5.5 ± 0.19 | 5.11 ± 0.29 | 5.18 ± 0.12 |
| 9d | >16 | >16 | >16 |
| 9e | >16 | >16 | 10.26 ± 0.20 |
| 9f | >16 | >16 | >16 |
| 9g | >16 | >16 | >16 |
| 9h | >16 | >16 | >16 |
| 9i | >16 | >16 | >16 |
| 9j | >16 | >16 | >16 |
| 9k | >16 | >16 | >16 |
| 9l | 10.4 ± 0.07 | 8.57 ± 0.51 | 10.8 ± 0.13 |
| 9m | >16 | >16 | >16 |
| 9n | 11.8 ± 0.54 | 10.49 ±0.16 | >16 |
| 9o | >16 | >16 | >16 |
| 9p | >16 | >16 | >16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascioferro, S.; Li Petri, G.; Parrino, B.; El Hassouni, B.; Carbone, D.; Arizza, V.; Perricone, U.; Padova, A.; Funel, N.; Peters, G.J.; et al. 3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma. Molecules 2020, 25, 329. https://doi.org/10.3390/molecules25020329
Cascioferro S, Li Petri G, Parrino B, El Hassouni B, Carbone D, Arizza V, Perricone U, Padova A, Funel N, Peters GJ, et al. 3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma. Molecules. 2020; 25(2):329. https://doi.org/10.3390/molecules25020329
Chicago/Turabian StyleCascioferro, Stella, Giovanna Li Petri, Barbara Parrino, Btissame El Hassouni, Daniela Carbone, Vincenzo Arizza, Ugo Perricone, Alessandro Padova, Niccola Funel, Godefridus J. Peters, and et al. 2020. "3-(6-Phenylimidazo [2,1-b][1,3,4]thiadiazol-2-yl)-1H-Indole Derivatives as New Anticancer Agents in the Treatment of Pancreatic Ductal Adenocarcinoma" Molecules 25, no. 2: 329. https://doi.org/10.3390/molecules25020329