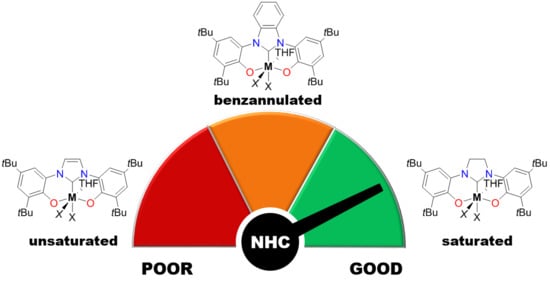
Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Bis-Phenolate NHC Complexes of Titanium and Hafnium
2.2. Copolymerization of CHO with CO2
3. Materials and Methods
3.1. Experimental Details
3.2. Synthesis of ([κ3-O,C,O]-INHC)TiCl(OiPr)(THF) 1a
3.3. Synthesis of ([κ3-O,C,O]-BzNHC)TiCl(OiPr) 1b
3.4. Synthesis of [κ3-O,C,O]-(INHC)Ti(OiPr)2 2a
3.5. Synthesis of ([κ3-O,C,O]-BzNHC)Ti(OiPr)2 2b
3.6. Synthesis of ([κ3-O,C,O]-BzNHC)HfCl(OiPr)(THF) 3b
3.7. Synthesis of ([κ3-O,C,O]-BzNHC)Hf(OiPr)2(THF) 4b
3.8. Copolymerization of CHO and CO2
3.9. X-ray Crystallographic Details
3.10. Computational Methods
3.10.1. Geometry Optimization
3.10.2. Single-Point Energy Calculations
3.10.3. Natural Bond Orbital Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Díez-González, S.; Nolan, S.P. Stereoelectronic Parameters Associated with N-heterocyclic Carbene (NHC) Ligands: A Quest for Understanding. Coord. Chem. Rev. 2007, 251, 874–883. [Google Scholar] [CrossRef]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Lin, I.J.B. Coinage Metal−N-heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. [Google Scholar] [CrossRef]
- Poyatos, M.; Mata, J.A.; Peris, E. Complexes with Poly(N-heterocyclic carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-heterocyclic Carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Riener, K.; Haslinger, S.; Raba, A.; Högerl, M.P.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. Chemistry of Iron N-Heterocyclic Carbene Complexes: Syntheses, Structures, Reactivities, and Catalytic Applications. Chem. Rev. 2014, 114, 5215–5272. [Google Scholar] [CrossRef]
- Nasr, A.; Winkler, A.; Tamm, M. Anionic N-Heterocyclic Carbenes: Synthesis, Coordination Chemistry and Applications in Homogeneous Catalysis. Coord. Chem. Rev. 2016, 316, 68–124. [Google Scholar] [CrossRef]
- Liddle, S.T.; Edworthy, I.S.; Arnold, P.L. Anionic Tethered N-heterocyclic Carbene Chemistry. Chem. Soc. Rev. 2007, 36, 1732–1744. [Google Scholar] [CrossRef]
- Pugh, D.; Danopoulos, A.A. Metal Complexes with ‘Pincer’-type Ligands Incorporating N-Heterocyclic Carbene Functionalities. Coord. Chem. Rev. 2007, 251, 610–641. [Google Scholar] [CrossRef]
- McGuinness, D. Alkene Oligomerisation and Polymerisation with Metal-NHC Based Catalysts. Dalton Trans. 2009, 2009, 6915–6923. [Google Scholar] [CrossRef] [PubMed]
- Bellemin-Laponnaz, S.; Dagorne, S. Group 1 and 2 and Early Transition Metal Complexes Bearing N-Heterocyclic Carbene Ligands: Coordination Chemistry, Reactivity, and Applications. Chem. Rev. 2014, 114, 8747–8774. [Google Scholar] [CrossRef]
- Hameury, S.; de Fremont, P.; Braunstein, P. Metal Complexes with Oxygen-functionalized NHC Ligands: Synthesis and Applications. Chem. Soc. Rev. 2017, 46, 632–733. [Google Scholar] [CrossRef]
- Zhang, D.; Zi, G. N-Heterocyclic Carbene (NHC) Complexes of Group 4 Transition Metals. Chem. Soc. Rev. 2015, 44, 1898–1921. [Google Scholar]
- Romain, C.; Bellemin-Laponnaz, S.; Dagorne, S. Recent Progress on NHC-stabilized Early Transition Metal (group 3–7) Complexes: Synthesis and Applications. Coord. Chem. Rev. 2020, 422, 213411. [Google Scholar]
- Kuhl, O. The Chemistry of Functionalised N-Heterocyclic Carbenes. Chem. Soc. Rev. 2007, 36, 592–607. [Google Scholar] [CrossRef]
- Charra, V.; de Frémont, P.; Braunstein, P. Multidentate N-Heterocyclic Carbene Complexes of the 3d Metals: Synthesis, Structure, Reactivity and Catalysis. Coord. Chem. Rev. 2017, 341, 53–176. [Google Scholar]
- Aihara, H.; Matsuo, T.; Kawaguchi, H. Titanium N-Heterocyclic Carbene Complexes Incorporating an Imidazolium-linked Bis(phenol). Chem. Commun. 2003, 2204–2205. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Gibson, V.C.; Steed, J.W. Bis(carbene)pyridine Complexes of the Early to Middle Transition Metals: Survey of Ethylene Oligomerization and Polymerization Capability. Organometallics 2004, 23, 6288–6292. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, N. Titanium Complexes Bearing Bisaryloxy-N-heterocyclic Carbenes: Synthesis, Reactivity, and Ethylene Polymerization Study. Organometallics 2009, 28, 499–505. [Google Scholar] [CrossRef]
- Bocchino, C.; Napoli, M.; Costabile, C.; Longo, P. Synthesis of Octahedral Zirconium Complex Bearing [NHC-O] Ligands, and its Behavior as Catalyst in the Polymerization of Olefins. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 862–870. [Google Scholar] [CrossRef]
- El-Batta, A.; Waltman, A.W.; Grubbs, R.H. Bis-ligated Ti and Zr Complexes of Chelating N-Heterocyclic Carbenes. J. Organomet. Chem. 2011, 696, 2477–2481. [Google Scholar] [CrossRef]
- Larocque, T.G.; Badaj, A.C.; Dastgir, S.; Lavoie, G.G. New Stable Aryl-substituted Acyclic Imino-N-heterocyclic Carbene: Synthesis, Characterisation and Coordination to Early Transition Metals. Dalton Trans. 2011, 40, 12705–12712. [Google Scholar] [CrossRef]
- Larocque, T.G.; Lavoie, G.G. Coordination and Reactivity Study of Titanium Phenoxo Complexes Containing a Bulky Bidentate Imino-N-heterocyclic Carbene Ligand. J. Organomet. Chem. 2012, 715, 26–32. [Google Scholar] [CrossRef]
- Dagorne, S.; Bellemin-Laponnaz, S.; Romain, C. Neutral and Cationic N-Heterocyclic Carbene Zirconium and Hafnium Benzyl Complexes: Highly Regioselective Oligomerization of 1-Hexene with a Preference for Trimer Formation. Organometallics 2013, 32, 2736–2743. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Henling, L.M.; Labinger, J.A.; Bercaw, J.E. Addition of a Phosphine Ligand Switches an N-Heterocyclic Carbene-zirconium Catalyst from Oligomerization to Polymerization of 1-Hexene. Dalton Trans. 2013, 42, 15544–15547. [Google Scholar] [CrossRef] [Green Version]
- Despagnet-Ayoub, E.; Takase, M.K.; Henling, L.M.; Labinger, J.A.; Bercaw, J.E. Mechanistic Insights on the Controlled Switch from Oligomerization to Polymerization of 1-Hexene Catalyzed by an NHC-Zirconium Complex. Organometallics 2015, 34, 4707–4716. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Zhang, D. Brønsted Base-Induced Rearrangement and Nucleophilic Addition of O/N-Functionalized NHCs and Relative Group 4 Metal Complexes for Ethylene Polymerization Catalysis. Organometallics 2016, 35, 138–150. [Google Scholar] [CrossRef]
- Quadri, C.C.; Lalrempuia, R.; Frøystein, N.Å.; Törnroos, K.W.; Le Roux, E. Steric Factors on Unsymmetrical O-Hydroxyaryl N-Heterocyclic Carbene Ligands Prevailing the Stabilization of Single Stereoisomer of bis-Ligated Titanium Complexes. J. Organomet. Chem. 2018, 860, 106–116. [Google Scholar] [CrossRef]
- Cho, J.; Hollis, T.K.; Helgert, T.R.; Valente, E.J. An Improved Method for the Synthesis of Zirconium (CCC-N-Heterocyclic Carbene) Pincer Complexes and Applications in Hydroamination. Chem. Commun. 2008, 5001–5003. [Google Scholar] [CrossRef]
- Cho, J.; Hollis, T.K.; Valente, E.J.; Trate, J.M. CCC–N-Heterocyclic Carbene Pincer Complexes: Synthesis, Characterization and Hydroamination Activity of a Hafnium Complex. J. Organomet. Chem. 2011, 696, 373–377. [Google Scholar] [CrossRef]
- Helgert, T.R.; Hollis, T.K.; Valente, E.J. Synthesis of Titanium CCC-NHC Pincer Complexes and Catalytic Hydroamination of Unactivated Alkenes. Organometallics 2012, 31, 3002–3009. [Google Scholar] [CrossRef]
- Barroso, S.; de Aguiar, S.R.M.M.; Munha, R.F.; Martins, A.M. New Zirconium Complexes Supported by N-Heterocyclic Carbene (NHC) Ligands: Synthesis and Assessment of Hydroamination Catalytic Properties. J. Organomet. Chem. 2014, 760, 60–66. [Google Scholar] [CrossRef]
- Clark, W.D.; Cho, J.; Valle, H.U.; Hollis, T.K.; Valente, E.J. Metal and Halogen Dependence of the Rate Effect in Hydroamination/Cyclization of Unactivated Aminoalkenes: Synthesis, Characterization, and Catalytic Rates of CCC-NHC Hafnium and Zirconium Pincer Complexes. J. Organomet. Chem. 2014, 751, 534–540. [Google Scholar] [CrossRef]
- Clark, W.D.; Leigh, K.N.; Webster, C.E.; Hollis, T.K. Experimental and Computational Studies of the Mechanisms of Hydroamination/Cyclisation of Unactivated α,ω-Amino-alkenes with CCC-NHC Pincer Zr Complexes*. Aust. J. Chem. 2016, 69, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Valle, H.U.; Akurathi, G.; Cho, J.; Clark, W.D.; Chakraborty, A.; Hollis, T.K. CCC-NHC Pincer Zr Diamido Complexes: Synthesis, Characterisation, and Catalytic Activity in Hydroamination/Cyclisation of Unactivated Amino-Alkenes, -Alkynes, and Allenes. Aust. J. Chem. 2016, 69, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Liddle, S.T.; Mungur, S.A.; Rodden, M.; Blake, A.J.; Arnold, P.L. Bifunctional Yttrium(III) and Titanium(IV) NHC Catalysts for Lactide Polymerisation. Chem. Commun. 2006, 1124–1126. [Google Scholar] [CrossRef]
- Romain, C.; Brelot, L.; Bellemin-Laponnaz, S.; Dagorne, S. Synthesis and Structural Characterization of a Novel Family of Titanium Complexes Bearing a Tridentate Bis-phenolate-N-Heterocyclic Carbene Dianionic Ligand and Their Use in the Controlled ROP of rac-Lactide. Organometallics 2010, 29, 1191–1198. [Google Scholar] [CrossRef]
- Romain, C.; Heinrich, B.; Laponnaz, S.B.; Dagorne, S. A Robust Zirconium N-Heterocyclic Carbene Complex for the Living and Highly Stereoselective Ring-opening Polymerization of rac-Lactide. Chem. Commun. 2012, 48, 2213–2215. [Google Scholar] [CrossRef]
- Zhao, N.; Hou, G.; Deng, X.; Zi, G.; Walter, M.D. Group 4 Metal Complexes with New Chiral Pincer NHC-ligands: Synthesis, Structure and Catalytic Activity. Dalton Trans. 2014, 43, 8261–8272. [Google Scholar] [CrossRef] [Green Version]
- Quadri, C.C.; Le Roux, E. Copolymerization of Cyclohexene Oxide with CO2 Catalyzed by Tridentate N-Heterocyclic Carbene Titanium(IV) Complexes. Dalton Trans. 2014, 43, 4242–4246. [Google Scholar] [PubMed] [Green Version]
- Hessevik, J.; Lalrempuia, R.; Nsiri, H.; Tornroos, K.W.; Jensen, V.R.; Le Roux, E. Sterically (un)Encumbered mer-Tridentate N-heterocyclic Carbene Complexes of Titanium(IV) for the Copolymerization of Cyclohexene Oxide with CO2. Dalton Trans. 2016, 45, 14734–14744. [Google Scholar] [PubMed]
- Quadri, C.C.; Lalrempuia, R.; Hessevik, J.; Törnroos, K.W.; Le Roux, E. Structural Characterization of Tridentate N-Heterocyclic Carbene Titanium(IV) Benzyloxide, Silyloxide, Acetate, and Azide Complexes and Assessment of Their Efficacies for Catalyzing the Copolymerization of Cyclohexene Oxide with CO2. Organometallics 2017, 36, 4477–4489. [Google Scholar]
- Lalrempuia, R.; Breivik, F.; Törnroos, K.W.; Le Roux, E. Coordination Behavior of Bis-phenolate Saturated and Unsaturated N-Heterocyclic Carbene Ligands to Zirconium: Reactivity and Activity in the Copolymerization of Cyclohexene Oxide with CO2. Dalton Trans. 2017, 46, 8065–8076. [Google Scholar]
- Lalrempuia, R.; Underhaug, J.; Törnroos, K.W.; Le Roux, E. Anionic Hafnium Species: An Active Catalytic Intermediate for the Coupling of Epoxides with CO2? Chem. Commun. 2019, 55, 7227–7230. [Google Scholar]
- Coates, G.W.; Moore, D.R. Discrete Metal-Based Catalysts for the Copolymerization of CO2 and Epoxides: Discovery, Reactivity, Optimization, and Mechanism. Angew. Chem. Int. Ed. 2004, 43, 6618–6639. [Google Scholar]
- Darensbourg, D.J.; Mackiewicz, R.M.; Phelps, A.L.; Billodeaux, D.R. Copolymerization of CO2 and Epoxides Catalyzed by Metal Salen Complexes. Acc. Chem. Res. 2004, 37, 836–844. [Google Scholar]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar]
- Kember, M.R.; Buchard, A.; Williams, C.K. Catalysts for CO2/Epoxide Copolymerisation. Chem. Commun. 2011, 47, 141–163. [Google Scholar]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent Advances in CO2/Epoxide Copolymerization—New Strategies and Cooperative Mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening Copolymerization (ROCOP): Synthesis and Properties of Polyesters and Polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of Carbon Dioxide and Epoxides by Metal Coordination Complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar]
- Le Roux, E. Chapter 6 Titanium-based Catalysts for Polymer Synthesis. In Sustainable Catalysis: With Non-endangered Metals, Part 1; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 116–139. [Google Scholar]
- Le Roux, E. Recent Advances on Tailor-made Titanium Catalysts for Biopolymer Synthesis. Coord. Chem. Rev. 2016, 306, 65–85. [Google Scholar]
- Mandal, M. Group 4 Complexes as Catalysts for the Transformation of CO2 into Polycarbonates and Cyclic Carbonates. J. Organomet. Chem. 2020, 907, 121067. [Google Scholar]
- Nakano, K.; Kobayashi, K.; Nozaki, K. Tetravalent Metal Complexes as a New Family of Catalysts for Copolymerization of Epoxides with Carbon Dioxide. J. Am. Chem. Soc. 2011, 133, 10720–10723. [Google Scholar]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Coupling Reaction Between CO2 and Cyclohexene Oxide: Selective Control from Cyclic Carbonate to Polycarbonate by Ligand Design of Salen/Salalen Titanium Complexes. Catal. Sci. Technol. 2014, 4, 3964–3972. [Google Scholar]
- Wang, Y.; Qin, Y.; Wang, X.; Wang, F. Trivalent Titanium Salen Complex: Thermally Robust and Highly Active Catalyst for Copolymerization of CO2 and Cyclohexene Oxide. ACS Catal. 2015, 5, 393–396. [Google Scholar]
- Mandal, M.; Chakraborty, D. Group 4 Complexes Bearing Bis(salphen) Ligands: Synthesis, Characterization, and Polymerization Studies. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 809–824. [Google Scholar]
- Mandal, M.; Monkowius, U.; Chakraborty, D. Synthesis and Structural Characterization of Titanium and Zirconium Complexes Containing Half-salen Ligands as Catalysts for Polymerization Reactions. New J. Chem. 2016, 40, 9824–9839. [Google Scholar]
- Garden, J.A.; White, A.J.P.; Williams, C.K. Heterodinuclear Titanium/Zinc Catalysis: Synthesis, Characterization and Activity for CO2/Epoxide Copolymerization and Cyclic Ester Polymerization. Dalton Trans. 2017, 46, 2532–2541. [Google Scholar]
- Raman, S.K.; Deacy, A.C.; Pena Carrodeguas, L.; Reis, N.V.; Kerr, R.W.F.; Phanopoulos, A.; Morton, S.; Davidson, M.G.; Williams, C.K. Ti(IV)–tris(phenolate) Catalyst Systems for the Ring-opening Copolymerization of Cyclohexene Oxide and Carbon Dioxide. Organometallics 2020, 39, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Su, C.K.; Chuang, H.J.; Li, C.Y.; Yu, C.Y.; Ko, B.T.; Chen, J.D.; Chen, M.J. Oxo-Bridged Bimetallic Group 4 Complexes Bearing Amine-bis(benzotriazole phenolate) Derivatives as Bifunctional Catalysts for Ring-opening Polymerization of Lactide and Copolymerization of Carbon Dioxide with Cyclohexene Oxide. Organometallics 2014, 33, 7091–7100. [Google Scholar] [CrossRef]
- Chuang, H.J.; Ko, B.T. Facilely Synthesized Benzotriazole Phenolate Zirconium Complexes as Versatile Catalysts for Copolymerization of Carbon Dioxide with Cyclohexene Oxide and Lactide Polymerization. Dalton Trans. 2015, 44, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Chakraborty, D.; Ramkumar, V. Zr(IV) Complexes Containing Salan-type Ligands: Synthesis, Structural Characterization and Role as Catalysts Towards the Polymerization of ε-Caprolactone, rac-Lactide, Ethylene, Homopolymerization and Copolymerization of Epoxides with CO2. RSC Adv. 2015, 5, 28536–28553. [Google Scholar] [CrossRef]
- Mandal, M.; Ramkumar, V.; Chakraborty, D. Salen Complexes of Zirconium and Hafnium: Synthesis, Structural Characterization and Polymerization Studies. Polym. Chem. 2019, 10, 3444–3460. [Google Scholar] [CrossRef]
- Borré, E.; Dahm, G.; Aliprandi, A.; Mauro, M.; Dagorne, S.; Bellemin-Laponnaz, S. Tridentate Complexes of Group 10 Bearing Bis-aryloxide N-Heterocyclic Carbene Ligands: Synthesis, Structural, Spectroscopic, and Computational Characterization. Organometallics 2014, 33, 4374–4384. [Google Scholar] [CrossRef]
- Harris, C.F.; Bayless, M.B.; van Leest, N.P.; Bruch, Q.J.; Livesay, B.N.; Bacsa, J.; Hardcastle, K.I.; Shores, M.P.; de Bruin, B.; Soper, J.D. Redox-Active Bis(phenolate) N-Heterocyclic Carbene [OCO] Pincer Ligands Support Cobalt Electron Transfer Series Spanning Four Oxidation States. Inorg. Chem. 2017, 56, 12421–12435. [Google Scholar] [CrossRef]
- Tapu, D.; Dixon, D.A.; Roe, C. 13C NMR Spectroscopy of “Arduengo-type” Carbenes and Their Derivatives. Chem. Rev. 2009, 109, 3385–3407. [Google Scholar] [CrossRef]
- Zhang, D. Dinuclear Titanium(IV) Complexes Bearing Phenoxide-Tethered N-Heterocyclic Carbene Ligands with cisoid Conformation through Control of Hydrolysis. Eur. J. Inorg. Chem. 2007, 2007, 4839–4845. [Google Scholar]
- Occhipinti, G.; Bjørsvik, H.R.; Jensen, V.R. Quantitative Structure−Activity Relationships of Ruthenium Catalysts for Olefin Metathesis. J. Am. Chem. Soc. 2006, 128, 6952–6964. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. Quantifying and Understanding the Electronic Properties of N-Heterocyclic Carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.V. Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
- Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M(NHC) (NHC = N-heterocyclic carbene) Bond. Coord. Chem. Rev. 2009, 253, 687–703. [Google Scholar] [CrossRef]
- Shukla, P.; Johnson, J.A.; Vidovic, D.; Cowley, A.H.; Abernethy, C.D. Amine Elimination Synthesis of a Titanium(IV) N-heterocyclic Carbene Complex with Short Intramolecular Cl⋯Ccarbene contacts. Chem. Commun. 2004, 360–361. [Google Scholar] [CrossRef]
- Jacobsen, H.; Correa, A.; Costabile, C.; Cavallo, L. π-Acidity and π-Basicity of N-Heterocyclic Carbene Ligands. A Computational Assessment. J. Organomet. Chem. 2006, 691, 4350–4358. [Google Scholar] [CrossRef]
- Tonner, R.; Heydenrych, G.; Frenking, G. Bonding Analysis of N-Heterocyclic Carbene Tautomers and Phosphine Ligands in Transition-Metal Complexes: A Theoretical Study. Chem. Asian J. 2007, 2, 1555–1567. [Google Scholar] [CrossRef]
- Horrer, G.; Krahfuß, M.J.; Lubitz, K.; Krummenacher, I.; Braunschweig, H.; Radius, U. N-Heterocyclic Carbene and Cyclic (Alkyl)(amino)carbene Complexes of Titanium(IV) and Titanium(III). Eur. J. Inorg. Chem. 2020, 2020, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen-sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Chemistry of Carbon Dioxide Relevant to Its Utilization: A Personal Perspective. Inorg. Chem. 2010, 49, 10765–10780. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Welter, R.; Brelot, L.; Dagorne, S. Synthesis and Structure of V(V) and Mn(III) NHC Complexes Supported by a Tridentate Bis-aryloxide-N-heterocyclic Carbene Ligand. J. Organomet. Chem. 2009, 694, 604–606. [Google Scholar] [CrossRef]
- Romain, C.; Specklin, D.; Miqueu, K.; Sotiropoulos, J.M.; Fliedel, C.; Bellemin-Laponnaz, S.; Dagorne, S. Unusual Benzyl Migration Reactivity in NHC-Bearing Group 4 Metal Chelates: Synthesis, Characterization, and Mechanistic Investigations. Organometallics 2015, 34, 4854–4863. [Google Scholar] [CrossRef]
- Martinsen, A.; Songstad, J. Preparation and Properties of Some Bis(triphenylphosphine)-iminium Salts, [[Ph3P)2N]X. Acta Chem. Scand. 1977, 31, 645–650. [Google Scholar] [CrossRef]
- APEX2. Version 2014.11-0; Bruker-AXS, Inc.: Madison, WI, USA, 2014.
- SAINT. Version 7.68A; Bruker-AXS, Inc.: Madison, WI, USA, 2010.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. XS. Version 2013/1; Georg-August-Universität Göttingen: Göttingen, Germany, 2013. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-group and Crystal-structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in 1977, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The Atoms Aluminum through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef] [Green Version]
- Feller, D. The Role of Databases in Support of Computational Chemistry Calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community Database for Computational Sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, K.A.; Figgen, D.; Dolg, M.; Stoll, H. Energy-consistent Relativistic Pseudopotentials and Correlation Consistent Basis Sets for the 4d Elements Y–Pd. J. Chem. Phys. 2007, 126, 124101. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron Affinities of the First-row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |







| (INHC)TiCl(OiPr) (THF) (1a) | (BzNHC)TiCl(OiPr)(THF) (1b-THF) a | (IsNHC)TiCl (OiPr)(THF) (1c) b | |
|---|---|---|---|
| Bond Length (Å)c | |||
| Ti-Ccarbene | 2.1310(13)/2.144 | 2.221(2)/2.214 | 2.166(3)/2.180 |
| Ti-Cl | 2.3739(4)/2.337 | 2.3459(8)/2.325 | 2.383(1)/2.336 |
| Ti-OiPr | 1.7671(11)/1.785 | 1.758(2)/1.789 | 1.779(2)/1.774 |
| Ti-THF | 2.2573(11)/2.318 | 2.2865(2)/2.347 | 2.272(2)/2.351 |
| Angle (°)c | |||
| ∠OAr-Ti-OAr | 159.14(4)/157.93 | 159.18(8)/158.70 | 159.19(9)/158.23 |
| ∠Ti-O-CiPr | 139.95(9)/143.18 | 160.5(3)/140.92 | 154.7(3)/160.48 |
| ∠N-Ccarbene-N | 105.22(11)/105.40 | 106.0(2)/106.20 | 108.8(2)/108.91 |
| Torsion Angle (°)c | |||
| ∠OAr-CAr-N-Ccarbene | 5.86/8.02 | 27.04/22.78 | 3.35/4.61 |
| ∠OAr-CAr-N-Ccarbene | 6.89/9.71 | −25.40/−25.67 | 3.51/5.54 |
| Complex | Ti-NHC Snapping Energy a (kcal mol−1) | NHC Fragment Charge b (e−) | NHC→Ti Net Donation c (№ of e−) |
|---|---|---|---|
| 1a | 597.3 | −0.76 | 0.57 |
| 1b-THF | 587.3 | −0.78 | 0.58 |
| 1c | 592.7 | −0.76 | 0.58 |

| Orbital a | MaH2 b | MbH2 | McH2 | ||
|---|---|---|---|---|---|
| C σ |  | Population (№ of electrons) | 1.71 | 1.74 | 1.69 |
| Energy (kcal mol−1) | −136.2 | −142.0 | −133.3 | ||
| C-N π |  | Population (№ of electrons) | 1.85 | 1.86 | 1.90 |
| Energy (kcal mol−1) | −200.4 | −200.3 | −198.3 | ||
| C-N π* |  | Population (№ of electrons) | 0.49 | 0.45 | 0.42 |
| Energy (kcal mol−1) | −37.4 | −37.4 | −33.9 | ||
| Complex | Donor Orbital a | Acceptor Orbital | E2 (kcal mol−1) |
|---|---|---|---|
| C-N π | Ti dπ | 2.95 | |
| 1a | OiPr LP | C-N π* | 1.01 |
| OTHF LP | C-N π* | 1.26 | |
| C-N π | Ti dπ | 2.44 | |
| 1b-THF | OiPr LP | C-N π* | 0.30 |
| OTHF LP | C-N π* | 3.28 | |
| 1c | C-N π | Ti dπ | 2.75 |
| C-N π | C-N π* | 0.83 | |
| OiPr LP | C-N π* | 0.39 | |
| OTHF LP | C-N π* | 1.42 |

| Entry | Precursor a,b | Yield (%) c | Productivity (gPCHC molM−1 h−1) | TOF (h−1) d | Mn (kg mol−1) e | Ɖe |
|---|---|---|---|---|---|---|
| 1 | 1a | 37 | 2745 | 22 | 1.8 | 1.59 |
| 2 | 2a | 26 | 1940 | 15 | - g | - g |
| 3 | 1b | 49 | 3618 | 28 | 4.5 | 1.43 |
| 4 | 1bf | 19 | 6709 | 62 | 1.2 | 1.54 |
| 5 | 2b | 48 | 3557 | 24 | 4.0 | 1.44 |
| 6 | 1cf | 52 | 18,572 | 116 | 5.9 | 1.41 |
| 7 | 3b | 10 | 770 | 6 | - g | - g |
| 8 | 4b | 12 | 897 | 7 | - g | - g |
| 9 | 3cf | 49 | 17,426 | 116 | 9.0 | 1.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suresh, L.; Lalrempuia, R.; B. Ekeli, J.; Gillis-D’Hamers, F.; Törnroos, K.W.; Jensen, V.R.; Le Roux, E. Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2. Molecules 2020, 25, 4364. https://doi.org/10.3390/molecules25194364
Suresh L, Lalrempuia R, B. Ekeli J, Gillis-D’Hamers F, Törnroos KW, Jensen VR, Le Roux E. Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2. Molecules. 2020; 25(19):4364. https://doi.org/10.3390/molecules25194364
Chicago/Turabian StyleSuresh, Lakshmi, Ralte Lalrempuia, Jonas B. Ekeli, Francis Gillis-D’Hamers, Karl W. Törnroos, Vidar R. Jensen, and Erwan Le Roux. 2020. "Unsaturated and Benzannulated N-Heterocyclic Carbene Complexes of Titanium and Hafnium: Impact on Catalysts Structure and Performance in Copolymerization of Cyclohexene Oxide with CO2" Molecules 25, no. 19: 4364. https://doi.org/10.3390/molecules25194364