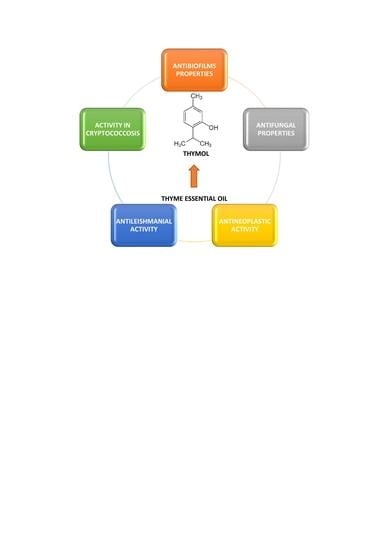
Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications
Abstract
:1. Introduction
2. Activity against Microbiological Biofilms
3. Antifungal Activity
3.1. Thymol Activity in Cryptococcosis
3.2. Other Antifungal Properties
4. Antileishmanial Properties
5. Antiviral Activity
5.1. Activity Against SARS-Cov-2
5.2. Other Antiviral Properties
6. Antineoplastic Activity
7. New Forms of Therapeutics Using Thyme Essential Oil and Its Active Ingredients
7.1. Natural Polymers
7.2. Semi-Synthetic Polymers
7.3. Microfibres
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.A.; Abbas-Mohammadi, M.; Barba, F.J.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules 2020, 25, 1152. [Google Scholar] [CrossRef] [Green Version]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; ISBN 928-718-505-0. [Google Scholar]
- European Medicines Agency. Assessment Report on Thymus vulgaris L., Thymus zygis Loefl. ex. L., aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis-loefl-ex-l-aetheroleum_en.pdf (accessed on 15 March 2020).
- European Medicines Agency. Assessment Report on Thymus vulgaris L., Thymus zygis L., herba. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis -l-herba_en.pdf (accessed on 15 March 2020).
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pawełczak, A.; Rolewska, K.; Węglarz, Z. Morphological and Chemical Traits as Quality Determinants of Common Thyme (Thymus vulgaris L.), on the Example of ‘Standard Winter’ Cultivar. Agronomy 2020, 10, 909. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Trindade, H. Sequencing and variation of terpene synthase gene (TPS2) as the major gene in biosynthesis of thymol in different Thymus species. Phytochemistry 2020, 169, 112126. [Google Scholar] [CrossRef] [PubMed]
- Bendif, H.; Peron, G.; Miara, M.D.; Sut, S.; Dall’Acqua, S.; Flamini, G.; Maggi, F. Total phytochemical analysis of Thymus munbyanus subsp. coloratus from Algeria by HS-SPME-GC-MS, NMR and HPLC-MSn studies. J. Pharmaceut. Biomed. 2020, 186, 113330. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thymol (accessed on 15 April 2020).
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Taee, H.A.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic Availability and Pharmacokinetics of Thymol in Humans. J. Clin. Pharmacol. 2002, 42, 731–737. [Google Scholar] [CrossRef]
- Nilima, T.; Silpi, B.; Rakesh, N.B.; Monali, R. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, 71–77. [Google Scholar]
- Walther, C.; Schmidtke, M. Anti-rhinovirus and anti-influenza virus activities of mucoactive secretolytic agents and plant extracts—A comparative in vitro study. Res. Sq. 2020; in press. [Google Scholar] [CrossRef]
- Eva, L.; Christin, M.; Ahmed, M.; Julia, D.; Pumaree, K.; Sharmistha, D.; Ute, C.; Lars-Norbert, P.; Stephan, P. Authorised medicinal product Aspecton® Oral Drops containing thyme extract KMTv24497 shows antiviral activity against viruses which cause respiratory infections. J. Herb. Med. 2018, 13, 26–33. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Li, Y.; Wena, J.; Du, C.; Hu, S.; Chen, J.; Zhang, S.; Zhang, N.; Gao, F.; Li, S.; Mao, X.; et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Codruta, H.S.; Lorena, F.; Oliviu, V.; Cristina, M.; Doina, M.; Adela, I.C.; Mirela, M. Essential Oil-Bearing Plants from Balkan Peninsula: Promising Sources for New Drug Candidates for the Prevention and Treatment of Diabetes Mellitus and Dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents Against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and Thymol: Strong Antimicrobial Agents Against Resistant Isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Hou, S.; Zhang, T.; Li, Z.; Liang, F. Distribution of ARGs and MGEs among glacial soil, permafrost, and sediment using metagenomic analysis. Environ. Pollut. 2018, 234, 339–346. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar]
- Al-Shuneigat, J.; Al-Sarayreh, S.; Al-Saraireh, Y.; Al-Qudah, M.; Al-Tarawneh, I. Effects of wild Thymus vulgaris essential oil on clinical isolates biofilm-forming bacteria. J. Dent. Med. Sci. 2014, 13, 62–66. [Google Scholar] [CrossRef]
- Bazargania, M.M.; Rohloff, J. Antibiofilm activity od essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Muzammil, S.; Aslam, B.; Siddique, M.H.; Saqalein, M.; Nisar, M.A. Quorum quenching: Role of nanoparticles as signal jammers in Gram-negative bacteria. Future Microbiol. 2019, 14, 61–72. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. WHO Pathogens Priority List Working Group. Discovery, research and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. (accessed on 15 March 2020).
- Qaralleh, H. Thymol Rich Thymbra capitata Essential Oil Inhibits Quorum Sensing, Virulence and Biofilm Formation of Beta Lactamase Producing Pseudomonas aeruginosa. Nat. Prod. Sci. 2019, 25, 172–180. [Google Scholar] [CrossRef]
- Kryvtsova, M.V.; Salamon, I.; Koscova, J.; Bucko, D.; Spivak, M. Antimicrobial, antibiofilm and biochemichal properties of Thymus vulgaris essential oil against clinical isolates of opportunistic infections. Biosyst. Divers. 2019, 27, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.P.; Perez, N.; Lozano, C.M.S.; Altube, M.J.; de Farias, M.A.; Portugal, R.V.; Buzzola, F.; Morilla, M.J.; Romero, E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicle. Phytomedicine 2019, 57, 339–351. [Google Scholar] [CrossRef]
- Nazar1, F.N.; Videla, E.A.; Marin, R.H. Thymol supplementation effects on adrenocortical, immune and biochemical variables recovery in Japanese quail after exposure to chronic heat stress. Animal 2019, 13, 318–325. [Google Scholar] [CrossRef]
- De Lira Mota, K.S.; de Oliveira Pereira, F.; de Oliveira, W.A.; Lima, I.O.; de Oliveira Lima, E. Antifungal Activity of Thymus vulgaris L. Essential Oil and its Constituent Phytochemicals against Rhizopus oryzae: Interaction with Ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Lioliosa, C.C.; Gortzib, O.; Lalasb, S.; Tsaknis, J.; Chinoua, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Tohidpour, A.; Sattari, M.; Omidbaigi, R.; Yadegar, A.; Nazemi, J. Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus. Phytomedicine 2010, 17, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Zhongwei, Y.; Yuyun, D.; Ping, O.; Tayyab, R.; Sajjad, H.; Tianyi, Z.; Zhongqiong, Y.; Hualin, F.; Juchun, L.; Changliang, H.; et al. Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms 2020, 8, 99. [Google Scholar]
- Kostoglou, D.; Protopappas, I.; Giaouris, E. Common Plant-Derived Terpenoids Present Increased Anti-Biofilm Potential against Staphylococcus Bacteria Compared to a Quaternary Ammonium Biocide. Foods 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef] [Green Version]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Mohamed, M.S.M.; Khalil, M.S.; Azmy, M.; Mabrouk, M.I. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumonia. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef]
- Virella, G. Mikrobiologia i Choroby Zakaźne, 1st ed.; Edra Urban & Partner: Wroclaw, Poland, 1999; pp. 388–389. [Google Scholar]
- Zavala, S.; Baddley, J.W. Cryptococcosis. Sem. Resp. Crit. Care M. 2020, 41, 69–79. [Google Scholar] [CrossRef]
- Rohatgi, S.; Pirofski, L.A. Host immunity to Cryptococcus neoformans. Future Microbiol. 2015, 10, 565–581. [Google Scholar] [CrossRef] [Green Version]
- Quintero, O.; Trachuk, P.; Lerner, M.Z.; Sarungbam, J.; Pirofski, L.P.; Park, S.O. Risk factors of laryngeal cryptococcosis: A case report. Med. Mycol. Case Rep. 2019, 24, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Biofilm formation by Cryptococcus neoformans. Microbiol. Spectr. 2015, 3, 1–11. [Google Scholar]
- Ajesh, K.; Sreejith, K. Cryptococcus laurentii biofilms: Structure, development and antifungal drug resistance. Mycopathologia 2012, 174, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Mishra, R.; Arora, N.; Chatrath, A.; Gangwar, R.; Roy, P.; Prasad, R. Antifungial and anti-biofilm activity of essential oil active components against Cryptococcus neoformans and Cryprococcus laurentii. Front. Microbiol. 2017, 8, 2161. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.V.N.; Capello, T.M.; Siqueira, L.J.A.; Lago, J.H.G.; Caseli, J. Mechanism of Action of Thymol on Cell Membranes Investigated through Lipid Monolayers at the Air–Water Interface and Molecular Simulation. Langmuir 2016, 32, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Poonam, K.; Neha, A.; Apurva, C.; Rashmi, G.; Vikas, P.; Poluri, K.M.; Prasad, R. Delineating the Biofilm Inhibition Mechanisms of Phenolic and Aldehydic Terpenes against Cryptococcus neoformans. ACS Omega 2019, 4, 17634–17648. [Google Scholar]
- Al-Shahrani, M.H.; Mahfoud, M.; Anvarbatcha, R.; Athar, M.T.; Al Asmari, A. Evaluation of antifungal activity and cytotoxicity of Thymus vulgaris essential oil. Pharmacogn. Commun. 2017, 7, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A Review In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 61–71. [Google Scholar]
- Avis, J.T.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microb. 2001, 67, 956–996. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.S.; Rhee, M.S. Short-Term Antifungal Treatments of Caprylic Acid with Carvacrol or Thymol Induce Synergistic 6-Log Reduction of Pathogenic Candida albicans by Cell Membrane Disruption and Efflux Pump Inhibition. Cell Physiol. Biochem. 2019, 53, 285–300. [Google Scholar]
- Ibrahim, H.M.S.; Baraka, M.A. Prevalence of intestinal protozoan parasitic infections among people attending Sebha Central Laboratory in Sebha, Libya: A retrospective study. Int. J. Appl. Sci. 2019, 1, 374–385. [Google Scholar]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 21 April 2020).
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kutateladze, T.G. Molecular structure analyses suggest strategies to therapeutically target SARS-CoV-2. Nat. Commun. 2020, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure function and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19). Available online: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19 (accessed on 24 August 2020).
- Hard-Surface Disinfectants and Hand Sanitizers (COVID-19): List of Disinfectants with Evidence for Use against COVID-19. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/list.html (accessed on 24 August 2020).
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, 1700436. [Google Scholar] [CrossRef]
- Toujani, M.M.; Rittà, M.; Civra, A.; Genovese, S.; Epifano, F.; Ghram, A.; Lembo, D.; Donalisio, M. Inhibition of HSV-2 infection by pure compounds from Thymus capitatus. Phytother. Res. 2018, 32, 1555–1563. [Google Scholar] [CrossRef]
- Word Health Organization. Cancer. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 April 2020).
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer Activity of Thymol: A Literature Based Review and Docking Study with Emphasis on its Anticancer Mechanisms. Iubmb Life 2019, 71, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of cancer. Bratisl. Med. J. 2020, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-H.; Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Jeong, J.-H.; Dong, X.; Lee, J.-W.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Anticancer Effect of Thymol on AGS Human Gastric Carcinoma Cells. J. Microbiol. Biotechnol. 2016, 26, 28–37. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Kiziltan, H.S. Effects of Thymol, a Natural Phenolic Compound, on Human Gastric Adenocarcinoma Cells In Vitro. Altern. Ther. Health Med. 2019, 25, 12–21. [Google Scholar] [PubMed]
- Pinna, R.; Filigheddu, E.; Juliano, C.; Palmieri, A.; Manconi, M.; D’hallewin, G.; Petretto, G.; Maioli, M.; Caddeo, C.; Manca, M.L.; et al. Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles. Pharmaceutics 2019, 11, 234. [Google Scholar] [CrossRef] [Green Version]
- Piombino, C.; Lange, H.; Sabuzi, F.; Galloni, P.; Conte, V.; Crestini, C. Lignosulfonate Microcapsules for Delivery and Controlled Release of Thymol and Derivatives. Molecules 2020, 25, 866. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Qiu, Y.; Chen, W.; Wei, Q. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mat. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef]
- Rassu, G.; Nieddu, M.; Bosi, P.; Trevisi, P.; Colombo, M.; Priori, D.; Manconi, P.; Giunchedi, P.; Gavini, E.; Boatto, G. Encapsulation and modified-release of thymol from oral microparticles as adjuvant or substitute to current medications. Phytomedicine 2014, 21, 1627–1632. [Google Scholar] [CrossRef]
- Zamani, Z.; Alipour, D.; Moghimi, H.R.; Mortazavi, S.A.R.; Saffary, M. Development and Evaluation of Thymol Microparticles Using Cellulose Derivatives as Controlled Release Dosage form. Iran J. Pharm. Res. 2015, 14, 1032–1040. [Google Scholar]
- Gámez, E.; Elizondo-Castillo, H.; Tascon, J.; García-Salinas, S.; Navascues, N.; Mendoza, G.; Arruebo, M.; Irusta, S. Antibacterial Effect of Thymol Loaded SBA-15 Nanorods Incorporated in PCL Electrospun Fibers. Nanomaterials 2020, 10, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, B.S.; Ghosh, S.K.; Patro, K.T.B. Preparation and characterization of famotidine microcapsule employing mucoadhesive polymers in combination to enhance gastro retention for oral delivery. Int. J. Pharm. 2009, 1, 112–120. [Google Scholar]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. https://doi.org/10.3390/molecules25184125
Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules. 2020; 25(18):4125. https://doi.org/10.3390/molecules25184125
Chicago/Turabian StyleKowalczyk, Adam, Martyna Przychodna, Sylwia Sopata, Agnieszka Bodalska, and Izabela Fecka. 2020. "Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications" Molecules 25, no. 18: 4125. https://doi.org/10.3390/molecules25184125