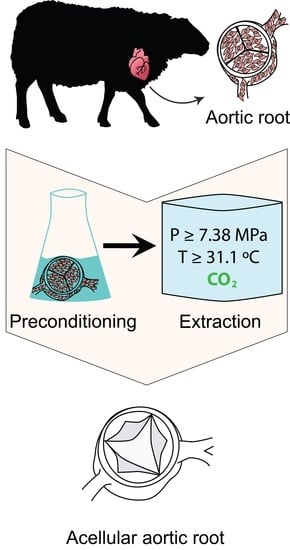
Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization
Abstract
:1. Introduction
2. Results
2.1. Ethanol-Based Decellularization
2.2. Alkali-Based Decellularization
2.3. Detergent-Based Decellularization
3. Discussion
4. Materials and Methods
4.1. Isolation of the Aortic Root
4.2. Aortic Root Decellularization
4.3. Histological Analysis
4.4. Confocal Laser Scanning Microscopy
4.5. Uniaxial Tensile Tests
4.6. DNA Quantification Test
4.7. MTT Test
4.8. Contact Cytotoxicity
4.9. Scanning Electron Microscopy
4.10. Atomic Force Microscopy
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sedrakyan, A.; Hebert, P.; Vaccarino, V.; Paltiel, A.D.; Elefteriades, J.A.; Mattera, J.; Lin, Z.; Roumanis, S.A.; Krumholz, H.M. Quality of life after aortic valve replacement with tissue and mechanical implants. J. Thorac. Cardiovasc. Surg. 2004, 128, 266–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesey, J.M.; Otto, C.M. Complications of prosthetic heart valves. Curr. Cardiol. Rep. 2004, 6, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C. Management and follow up of prosthetic heart valves. Heart 2004, 90, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gott, V.L.; Alejo, D.E.; Cameron, D.E. Mechanical heart valves: 50 years of evolution. Ann. Thorac. Surg. 2003, 76, S2230–S2239. [Google Scholar] [CrossRef] [PubMed]
- Cannegieter, S.C.; Rosendaal, F.R.; Briet, E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodés-Cabau, J. Aortic bioprosthetic valve durability: Incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J. Am. Coll. Surg. 2017, 70, 1013–1028. [Google Scholar] [CrossRef]
- Elkins, R.; Dawson, P.; Goldstein, S.; Walsh, S.; Black, K. Decellularized human valve allografts. Ann. Thorac. Surg. 2001, 71, S428–S432. [Google Scholar] [CrossRef]
- Tudorache, I.; Horke, A.; Cebotari, S.; Sarikouch, S.; Boethig, D.; Breymann, T.; Beerbaum, P.; Bertram, H.; Westhoff-Bleck, M.; Theodoridis, K.; et al. Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur. J. Cardiothorac. Surg. 2016, 50, 89–97. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Arenas-Herrera, J.E.; Ko, I.K.; Atala, A.; Yoo, J.J. Decellularization for whole organ bioengineering. Biomed. Mater. 2013, 8, 014106. [Google Scholar] [CrossRef]
- Bibevski, S.; Ruzmetov, M.; Fortuna, R.S.; Turrentine, M.W.; Brown, J.W.; Ohye, R.G. Performance of SynerGraft decellularized pulmonary allografts compared with standard cryopreserved allografts: Results from multiinstitutional data. Ann. Thorac. Surg. 2017, 103, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boethig, D.; Horke, A.; Hazekamp, M.; Meyns, B.; Rega, F.; Van Puyvelde, J.; Hübler, M.; Schmiady, M.; Ciubotaru, A.; Stellin, G.; et al. European study on decellularized homografts for pulmonary valve replacement: Initial results from the prospective ESPOIR Trial and ESPOIR Registry data. Eur. J. Cardiothorac. Surg. 2019, 56, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Salvatore, M.A.; Schup-Magoffin, P.J.; Hu, D.P.; Christman, K.L. Design and characterization of an injectable pericardial matrix gel: A potentially autologous scaffold for cardiac tissue engineering. Tissue Eng. Part A 2010, 16, 2017–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Chen, C.Z.; Wang, X.N.; Zhu, Y.B.; Gu, Y.J. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J. Biomed. Mater. Res. Part B 2009, 91, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Novelo, B.; Castellano, L.E.; Padilla-Miranda, R.G.; Lona-Ramos, M.C.; Cuéllar-Mata, P.; Vega-González, A.; Murguía-Pérez, M.; Mata-Mata, J.L.; Ávila, E.E. The component leaching from decellularized pericardial bioscaffolds and its implication in the macrophage response. J. Biomed. Mater. Res. Part A 2016, 104, 2810–2822. [Google Scholar] [CrossRef]
- Pagoulatou, E.; Triantaphyllidou, I.-E.; Vynios, D.H.; Papachristou, D.J.; Koletsis, E.; Deligianni, D.; Mavrilas, D. Biomechanical and structural changes following the decellularization of bovine pericardial tissues for use as a tissue engineering scaffold. J. Mater. Sci. Mater. Med. 2012, 23, 1387–1396. [Google Scholar] [CrossRef]
- Hülsmann, J.; Grün, K.; El Amouri, S.; Barth, M.; Hornung, K.; Holzfuß, C.; Lichtenberg, A.; Akhyari, P. Transplantation material bovine pericardium: Biomechanical and immunogenic characteristics after decellularization vs. glutaraldehyde-fixing. Xenotransplantation 2012, 19, 286–297. [Google Scholar] [CrossRef]
- Sun, W.Q.; Xu, H.; Sandor, M.; Lombardi, J. Process-induced extracellular matrix alterations affect the mechanisms of soft tissue repair and regeneration. J. Tissue Eng. 2013, 4, 2041731413505305. [Google Scholar] [CrossRef] [Green Version]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Cebotari, S.; Tudorache, I.; Jaekel, T.; Hilfiker, A.; Dorfman, S.; Ternes, W.; Haverich, A.; Lichtenberg, A. Detergent decellularization of heart valves for tissue engineering: Toxicological effects of residual detergents on human endothelial cells. Artif. Organs 2010, 34, 206–210. [Google Scholar] [CrossRef]
- Hwang, J.; San, B.H.; Turner, N.J.; White, L.J.; Faulk, D.M.; Badylak, S.F.; Li, Y.; Yu, S.M. Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta. Biomater. 2017, 53, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Terada, D.; Yamaoka, T.; Kitamura, S.; Fujisato, T. Cell removal with supercritical carbon dioxide for acellular artificial tissue. Chem. Technol. Biotechnol. 2008, 83, 943–949. [Google Scholar] [CrossRef]
- Sun, Y.P. Supercritical Fluid Technology in Materials Science and Engineering: Syntheses: Properties, and Applications; Taylor & Francis: Oxfordshire, UK, 2002; p. 600. [Google Scholar]
- Clifford, A.A.; Williams, J.R. Introduction to supercritical fluids and their applications. In Supercritical Fluid Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; p. 16. [Google Scholar]
- Peach, J.; Eastoe, J. Supercritical carbon dioxide: A solvent like no other. Beilstein J. Org. Chem. 2014, 10, 18781895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez, H.M.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsieh, D.-J.; Periasamy, S.; Chuang, C.-T.; Tseng, F.-W.; Kuo, J.-C.; Tarng, Y.-W. Regenerative porcine dermal collagen matrix developed by supercritical carbon dioxide extraction technology: Role in accelerated wound healing. Materialia 2019, 9, 100576. [Google Scholar] [CrossRef]
- Halfwerk, F.R.; Rouwkema, J.; Gossen, J.A.; Grandjean, J.G. Supercritical carbon dioxide decellularised pericardium: Mechanical and structural characterisation for applications in cardio-thoracic surgery. J. Mech. Behav. Biomed. Mater. 2018, 77, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Zambon, A.; Vetralla, M.; Urbani, L.; Pantano, M.F.; Ferrentino, G.; Pozzobon, M.; Pugno, N.M.; Coppi, P.D.; Elvassore, N.; Spilimbergo, S. Dry acellular oesophageal matrix prepared by supercritical carbon dioxide. J. Supercrit. Fluids 2016, 115, 33–41. [Google Scholar] [CrossRef]
- Casali, D.M.; Handleton, R.M.; Shazly, T.; Matthews, M.A. A novel supercritical CO2-based decellularization method for maintaining scaffold hydration and mechanical properties. J. Supercrit. Fluids 2018, 131, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.K.; Luo, B.; Guneta, V.; Li, L.; Foo, S.E.M.; Dai, Y.; Tan, T.T.Y.; Tan, N.S.; Choong, C.; Wong, M.T.C. Supercritical carbon dioxide extracted extracellular matrix material from adipose tissue. Mater. Sci. Eng. C 2017, 75, 349–358. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Tseng, F.-W.; Chang, W.-H.; Peng, I.-C.; Hsieh, D.-J.; Wu, S.-W.; Yeh, M.-L. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater. 2017, 58, 238–243. [Google Scholar] [CrossRef]
- Hennessy, R.S.; Jana, S.; Tefft, B.J.; Helder, M.R.; Young, M.D.; Hennessy, R.R.; Stoyles, N.J.; Lerman, A. Supercritical carbon dioxide–based sterilization of decellularized heart valves. Jacc Basic Transl. Sci. 2017, 2, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.S.; Go, J.L.; Hennessy, R.R.; Tefft, B.J.; Jana, S.; Stoyles, N.J.; Al-Hijji, M.A.; Thaden, J.J.; Pislaru, S.V.; Simari, R.D.; et al. Recellularization of a novel off-the-shelf valve following xenogenic implantation into the right ventricular outflow tract. PLoS ONE 2017, 12, e0181614. [Google Scholar] [CrossRef] [PubMed]
- Van de Pol, G.J.; Bonar, F.; Salmon, L.J.; Roe, J.P.; Pinczewski, L.A. Supercritical carbon dioxide–sterilized bone allograft in the treatment of tunnel defects in 2-stage revision anterior cruciate ligament reconstruction: A histologic evaluation. Arthroscopy 2018, 34, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Aslan, B.; Hosseinian, P.; Aydin, H.M. Supercritical carbon dioxide-assisted decellularization of aorta and cornea. Tissue Eng. Part C 2017, 23, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Grebenik, E.A.; Istranov, L.P.; Istranova, E.V.; Churbanov, S.N.; Shavkuta, B.S.; Dmitriev, R.I.; Veryasova, N.N.; Kotova, S.L.; Kurkov, A.V.; Shekhter, A.B.; et al. Chemical cross-linking of xenopericardial biomeshes: A bottom-up study of structural and functional correlations. Xenotransplantation 2019, 26, e12506. [Google Scholar] [CrossRef] [PubMed]
- Grebenik, E.A.; Gafarova, E.R.; Istranov, L.P.; Istranova, E.V.; Ma, X.; Xu, J.; Weisheng, G.; Atala, A.; Timashev, P.S. Mammalian pericardium-based bioprosthetic materials in xenotransplantation and tissue engineering. Biotechnol. J. 2020, e1900334. [Google Scholar] [CrossRef]
- Veryasova, N.N.; Lazhko, A.E.; Isaev, D.E.; Grebenik, E.A.; Timashev, P.S. Supercritical carbon dioxide—A powerful tool for green biomaterial chemistry. Russ. J. Phys. Chem. B 2019, 13, 1079–1087. [Google Scholar] [CrossRef]
- Herijgers, P.; Ozaki, S.; Verbeken, E.; Van Lommel, A.; Ràcz, R.; Zietkiewicz, M.; Perek, B.; Flameng, W. Calcification characteristics of porcine stentless valves in juvenile sheep. Eur. J. Cardiothorac. Surg. 1999, 15, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Sands, M.P.; Rittenhouse, E.A.; Mohri, H.; Merendino, K.A. An anatomical comparison of human, pig, calf, and sheep aortic valves. Ann. Thorac. Surg. 1969, 8, 407–414. [Google Scholar] [CrossRef]
- Kotova, S.L.; Timashev, P.S.; Guller, A.E.; Shekhter, A.B.; Misurkin, P.I.; Bagratashvili, V.N.; Solovieva, A.B. Collagen structure deterioration in the skin of patients with pelvic organ prolapse determined by atomic force microscopy. Microsc. Microanal. 2015, 21, 324–333. [Google Scholar] [CrossRef]
- Nobari, S.; Mongrain, R.; Leask, R.; Cartier, R. The effect of aortic wall and aortic leaflet stiffening on coronary hemodynamic: A fluid–structure interaction study. Med. Biol. Eng. Comput. 2013, 51, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Wyss, K.; Yip, C.Y.; Mirzaei, Z.; Jin, X.; Chen, J.H.; Simmons, C.A. The elastic properties of valve interstitial cells undergoing pathological differentiation. J. Biomech. 2012, 45, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Khorramirouz, R.; Sabetkish, S.; Akbarzadeh, A.; Muhammadnejad, A.; Heidari, R.; Kajbafzadeh, A.M. Effect of three decellularisation protocols on the mechanical behaviour and structural properties of sheep aortic valve conduits. Adv. Med. Sci. 2014, 59, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Melman, L.; Jenkins, E.D.; Hamilton, N.A.; Bender, L.C.; Brodt, M.D.; Deeken, C.R.; Greco, S.C.; Frisella, M.M.; Matthews, B.D. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia 2011, 15, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, A.; Yang, Y. Decellularization strategies for regenerative medicine: From processing techniques to applications. Biomed. Res. Int. 2017, 9831534, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpour, S.; Golshan, A.; Memarian, T. Comparative study on surface geometric properties of decellularized and glutaraldehyde fixed bovine pericardium and possible effects on cell seeding. Image 2018, 2, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Elagin, V.; Kuznetsova, D.; Grebenik, E.; Zolotov, D.A.; Istranov, L.; Zharikova, T.; Istranova, E.; Polozova, A.; Reunov, D.; Kurkov, A.; et al. Multiparametric optical bioimaging reveals the fate of epoxy crosslinked biomeshes in the mouse subcutaneous implantation model. Front. Bioeng. Biotechnol. 2020, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hornick, J.L. Practical Soft Tissue Pathology: A Diagnostic Approach E-Book: A Volume in the Pattern Recognition Series; Elsevier: Amsterdam, The Netherlands, 2017; p. 592. [Google Scholar]
- Rittié, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. In Fibrosis: Methods in Molecular Biology; Humana Press: Totowa, NY, USA, 2017; Volume 1627, pp. 395–407. [Google Scholar] [CrossRef]
Sample Availability: Samples of the tissues are available from the authors. |





| Treatment Method | Tissue | Thickness, mm | Direction | Ultimate Tensile Strength, MPa | Strain at Fracture, % | Young’s Modulus, MPa |
|---|---|---|---|---|---|---|
| None | Leaflet | 0.23 ± 0.04 | Circ.* | 4.19 ± 1.44 | 56.3 ± 14.4 | 12.01 ± 1.08 |
| Wall | 1.5 ± 0.35 | Circ. | 0.43 ± 0.20 | 29.4 ± 5.0 | 2.83 ± 1.16 | |
| Long.** | 0.35 ± 0.11 | 36.6 ± 15.1 | 2.71 ± 1.37 | |||
| scCO2 (15 MPa)-Ethanol | Leaflet | 0.13 ± 0.01 | Circ. | 5.99 ± 1.54 | 22.5 ± 11.7 | 109.13 ± 15.24 |
| Wall | 1.15 ± 0.17 | Circ. | 1.13 ± 0.20 | 55.0 ± 20.0 | 4.02 ± 2.02 | |
| Long. | 0.23 ± 0.04 | 18.5 ± 6.2 | 3.63 ± 0.93 | |||
| scCO2 (25 MPa)-Ethanol | Leaflet | 0.19 ± 0.04 | Circ. | 12.76 ± 6.09 | 31.5 ± 15.0 | 104.43 ± 29.57 |
| Wall | 1.66 ± 0.21 | Circ. | 1.01 ± 0.58 | 54.2 ± 14.4 | 3.85 ± 0.68 | |
| Long. | 0.31 ± 0.10 | 26.7 ± 12.2 | 3.99 ± 0.76 | |||
| Alkali-salt + scCO2 (10 MPa) | Leaflet | 0.10 ± 0.01 | Circ. | 24.50 ± 9.19 | 46.6 ± 25.5 | 2043.60 ± 903.98 |
| Wall | 1.30 ± 0.25 | Circ. | 0.46 ± 0.08 | 55.0 ± 25.0 | 1.04 ± 0.46 | |
| Long. | 0.14 ± 0.04 | 32.5 ± 7.5 | 0.90 ± 0.18 | |||
| Alkali-salt + scCO2 (15 MPa) | Leaflet | 0.10 ± 0.01 | Circ. | 47.38 ± 18.36 | 53.3 ± 24.4 | 4500.00 ± 2039.12 |
| Wall | 1.42 ± 0.33 | Circ. | 0.60 ± 0.13 | 36.3 ± 3.8 | 1.62 ± 0.49 | |
| Long. | 0.19 ± 0.01 | 37.5 ± 5.0 | 0.69 ± 0.04 | |||
| Detergent | Leaflet | 0.15 ± 0.03 | Circ. | 5.52 ± 2.28 | 68.4 ± 32.3 | 4.79 ± 2.25 |
| Wall | 1.26 ± 0.11 | Circ. | 0.48 ± 0.19 | 76.0 ± 21.7 | 0.31 ± 0.04 | |
| Long. | 1.27 ± 0.08 | 41.7 ± 17.2 | 2.46 ± 0.59 | |||
| Detergents + scCO2 (15 MPa) | Leaflet | 0.12 ± 0.03 | Circ. | 76.27 ± 27.24 | 15.0 ± 5.0 | 207.98 ± 38.54 |
| Wall | 1.41 ± 0.15 | Circ. | 0.39 ± 0.06 | 22.5 ± 8.3 | 1.40 ± 0.67 | |
| Long. | 0.15 ± 0.04 | 42.5 ± 10.0 | 0.44 ± 0.06 | |||
| Detergents + scCO2 (25 MPa) | Leaflet | 0.12 ± 0.01 | Circ. | 20.43 ± 8.54 | 25.0 ± 5.0 | 106.20 ± 56.11 |
| Wall | 1.16 ± 0.25 | Circ. | 0.87 ± 0.03 | 40.0 ± 10.0 | 1.15 ± 0.55 | |
| Long. | 0.15 ± 0.04 | 56.7 ± 32.2 | 0.51 ± 0.27 |
| Treatment Method | Ref. | Preconditioning | Extraction Parameters | Co-Solvent |
|---|---|---|---|---|
| scCO2-Ethanol | [22] | None | T = 37 °C, P = 15 MPa, t = 3 h | 95% Ethanol |
| T = 37 °C, P = 25 MPa, t = 3 h | 95% Ethanol | |||
| Alkali-salt + scCO2 | [28] | 1 M NaOH + 0.8 M Na2SO4 (1 h) | T = 37 °C, P = 10 MPa, t = 1 h | None |
| T = 37 °C, P = 15 MPa, t = 1 h | None | |||
| Detergent | [8] | 0.5% SDS/0.5% SD (12 h) | - | None |
| Detergent + scCO2 | [28,30] | 0.5% SDS/0.5% SD (12 h) | T = 37 °C, P = 15 MPa, t = 3 h | None |
| T = 37 °C, P = 25 MPa, t = 3 h | None |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafarova, E.R.; Grebenik, E.A.; Lazhko, A.E.; Frolova, A.A.; Kuryanova, A.S.; Kurkov, A.V.; Bazhanov, I.A.; Kapomba, B.S.; Kosheleva, N.V.; Novikov, I.A.; et al. Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization. Molecules 2020, 25, 3923. https://doi.org/10.3390/molecules25173923
Gafarova ER, Grebenik EA, Lazhko AE, Frolova AA, Kuryanova AS, Kurkov AV, Bazhanov IA, Kapomba BS, Kosheleva NV, Novikov IA, et al. Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization. Molecules. 2020; 25(17):3923. https://doi.org/10.3390/molecules25173923
Chicago/Turabian StyleGafarova, Elvira R., Ekaterina A. Grebenik, Alexey E. Lazhko, Anastasia A. Frolova, Anastasia S. Kuryanova, Alexandr V. Kurkov, Ilya A. Bazhanov, Byron S. Kapomba, Nastasia V. Kosheleva, Ivan A. Novikov, and et al. 2020. "Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization" Molecules 25, no. 17: 3923. https://doi.org/10.3390/molecules25173923