Spray Drying for the Encapsulation of Oils—A Review
Abstract
:1. Introduction
2. Oil Encapsulation Benefits
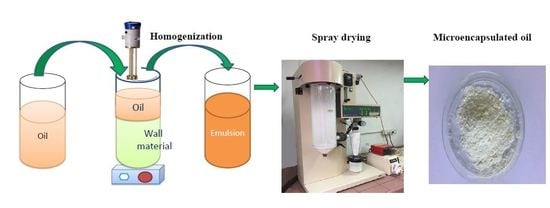
3. Encapsulation Using the Spray Drying Technique
4. Unit Operation of Spray Drying
4.1. Feed Atomization
4.2. Air Flow Contact
4.3. Drying and Particle Formation
4.4. Separation of Product from the Drying Air
5. Optimizing the Encapsulation Process Conditions
5.1. Inlet and Outlet Temperatures
5.2. Total Solids of the Emulsion
5.3. Wall Materials
5.3.1. Carbohydrates
5.3.2. Gums
5.3.3. Proteins
6. Applications of Encapsulated Oils in Food
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Marrelli, M.; Statti, G.; Conforti, F. A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders. Molecules 2020, 25, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giorgio, L.; Salgado, P.R.; Mauri, A.N. Encapsulation of Fish Oil in Soybean Protein Particles by Emulsification and Spray Drying. Food Hydrocoll. 2019, 87, 891–901. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent Advances in the Microencapsulation of Omega-3 Oil and Probiotic Bacteria through Complex Coacervation: A Review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Hasani, S. Characteristics and Oxidative Stability of Fish Oil Nano-Liposomes and Its Application in Functional Bread. J. Food Meas. Charact. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Agnihotri, N.; Mishra, R.; Goda, C.; Arora, M. Microencapsulation—A Novel Approach in Drug Delivery: A Review. Indo Glob. J. Pharm. Sci. 2012, 2, 1–20. [Google Scholar]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of Natural Antioxidants for Food Application—The Specific Case of Coffee Antioxidants—A Review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Lozano-Vazquez, G.; Lobato-Calleros, C.; Escalona-Buendia, H.; Chavez, G.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of the Weight Ratio of Alginate-Modified Tapioca Starch on the Physicochemical Properties and Release Kinetics of Chlorogenic Acid Containing Beads. Food Hydrocoll. 2015, 48, 301–311. [Google Scholar] [CrossRef]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent Advances in the Spray Drying Encapsulation of Essential Fatty Acids and Functional Oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Amstad, E. Capsules: Their Past and Opportunities for Their Future; ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- Estevinho, B.N.; Rocha, F. Application of Biopolymers in Microencapsulation Processes. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 191–222. [Google Scholar]
- Zuidam, N.J.; Nedović, V. Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Zheng, X.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Feng, Y. Developments in Taste-Masking Techniques for Traditional Chinese Medicines. Pharmaceutics 2018, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.-H.; Kim, Y.-P.; Lee, Y.-M.; Seo, E.-M.; Lee, K.-W.; Kim, H.-S. Optimization of Microencapsulation of Seed Oil by Response Surface Methodology. Food Chem. 2008, 107, 98–105. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Kolanowski, W.; Ziolkowski, M.; Weißbrodt, J.; Kunz, B.; Laufenberg, G. Microencapsulation of Fish Oil by Spray Drying—Impact on Oxidative Stability. Part 1. Eur. Food Res. Technol. 2006, 222, 336–342. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid. Based Complement. Altern. Med. 2014, 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Gupta, S.; Khan, S.; Muzafar, M.; Kushwaha, M.; Yadav, A.K.; Gupta, A.P. Encapsulation: Entrapping Essential Oil/Flavors/Aromas in Food. In Encapsulations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–268. [Google Scholar]
- Manaf, M.A.; Subuki, I.; Jai, J.; Raslan, R.; Mustapa, A.N. Encapsulation of Volatile Citronella Essential Oil by Coacervation: Efficiency and Release Study. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 12072. [Google Scholar]
- Encina, C.; Márquez-Ruiz, G.; Holgado, F.; Giménez, B.; Vergara, C.; Robert, P. Effect of Spray-Drying with Organic Solvents on the Encapsulation, Release and Stability of Fish Oil. Food Chem. 2018, 263, 283–291. [Google Scholar] [CrossRef]
- Melgosa, R.; Benito-Román, Ó.; Sanz, M.T.; de Paz, E.; Beltrán, S. Omega–3 Encapsulation by PGSS-Drying and Conventional Drying Methods. Particle Characterization and Oxidative Stability. Food Chem. 2019, 270, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, N.K.; Meor Hussin, A.S.; Tan, C.P.; Abdul Manap, M.Y.; Alhelli, A.M. Quality Changes of Microencapsulated Nigella sativa Oil upon Accelerated Storage. Int. J. Food Prop. 2017, 20, S2395–S2408. [Google Scholar] [CrossRef] [Green Version]
- Da Veiga, R.D.S.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential Oils Microencapsulated Obtained by Spray Drying: A Review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Stability of Microencapsulated Strawberry Flavour by Spray Drying, Freeze Drying and Fluid Bed. Powder Technol. 2019, 347, 179–185. [Google Scholar] [CrossRef]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Alves, A.R.C. Drying by Spray Drying in the Food Industry: Micro-Encapsulation, Process Parameters and Main Carriers Used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar]
- Ng, S.; Jessie, L.L.; Tan, C.; Long, K.; Nyam, K. Effect of Accelerated Storage on Microencapsulated Kenaf Seed Oil. J. Am. Oil Chem. Soc. 2013, 90, 1023–1029. [Google Scholar] [CrossRef]
- Schuck, P.; Dolivet, A.; Méjean, S.; Zhu, P.; Blanchard, E.; Jeantet, R. Drying by Desorption: A Tool to Determine Spray Drying Parameters. J. Food Eng. 2009, 94, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Edris, A.E.; Kalemba, D.; Adamiec, J.; Piątkowski, M. Microencapsulation of Nigella sativa Oleoresin by Spray Drying for Food and Nutraceutical Applications. Food Chem. 2016, 204, 326–333. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Carazo-Díaz, C.; Gabaldón, J.A.; Núñez-Delicado, E. Optimization of the Microencapsulation of Synthetic Strawberry Flavour with Different Blends of Encapsulating Agents Using Spray Drying. Powder Technol. 2018, 338, 591–598. [Google Scholar] [CrossRef]
- Carmona, P.A.O.; Garcia, L.C.; de Aquino Ribeiro, J.A.; Valadares, L.F.; de Figueiredo Marçal, A.; de França, L.F.; Mendonça, S. Effect of Solids Content and Spray-Drying Operating Conditions on the Carotenoids Microencapsulation from Pressed Palm Fiber Oil Extracted with Supercritical CO2. Food Bioprocess. Technol. 2018, 11, 1703–1718. [Google Scholar] [CrossRef]
- Başyiğit, B.; Sağlam, H.; Kandemir, Ş.; Karaaslan, A.; Karaaslan, M. Microencapsulation of Sour Cherry Oil by Spray Drying: Evaluation of Physical Morphology, Thermal Properties, Storage Stability, and Antimicrobial Activity. Powder Technol. 2020, 364, 654–663. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of Process Conditions on the Microencapsulation of Coffee Oil by Spray Drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Ng, S.-K.; Choong, Y.-H.; Tan, C.-P.; Long, K.; Nyam, K.-L. Effect of Total Solids Content in Feed Emulsion on the Physical Properties and Oxidative Stability of Microencapsulated Kenaf Seed Oil. LWT-Food Sci. Technol. 2014, 58, 627–632. [Google Scholar] [CrossRef]
- Shamaei, S.; Seiiedlou, S.S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of Walnut Oil by Spray Drying: Effects of Wall Material and Drying Conditions on Physicochemical Properties of Microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of Spray Drying Microencapsulation of Almond Oil into Taro Starch Spherical Aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Lavanya, M.N.; Kathiravan, T.; Moses, J.A.; Anandharamakrishnan, C. Influence of Spray-Drying Conditions on Microencapsulation of Fish Oil and Chia Oil. Dry. Technol. 2019, 279–292. [Google Scholar] [CrossRef]
- Castel, V.; Rubiolo, A.C.; Carrara, C.R. Brea Gum as Wall Material in the Microencapsulation of Corn Oil by Spray Drying: Effect of Inulin Addition. Food Res. Int. 2018, 103, 76–83. [Google Scholar] [CrossRef]
- Quispe, N.B.P.; Chaves, M.A.; Dos Santos, A.F.; Bastos, T.D.S.; Castro, S.S. Microencapsulation of Virgin Coconut Oil by Spray Drying/Microencapsulação de Óleo de Coco Virgem Por Spray Spray. Braz. J. Dev. 2020, 6, 1510–1529. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Erazo, E.M.; Consoli, L.; Hubinger, M.D. Spray Drying of Mono- and Double-Layer Emulsions of PUFA-Rich Vegetable Oil Homogenized by Ultrasound. Dry. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Linke, A.; Linke, T.; Hinrichs, J.; Kohlus, R. Factors Determining the Surface Oil Concentration of Encapsulated Lipid Particles—Impact of the Spray Drying Conditions. Dry. Technol. 2019, 1–14. [Google Scholar] [CrossRef]
- Pino, J.A.; Sosa-Moguel, O.; Sauri-Duch, E.; Cuevas-Glory, L. Microencapsulation of Winter Squash (Cucurbita moschata Duchesne) Seed Oil by Spray Drying. J. Food Process. Preserv. 2019, 43, e14136. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro, L.F.; Thomazini, M.; Pallone, E.M.J.A.; do Amaral Sobral, P.J.; de Castro, I.A.; Favaro-Trindade, C.S. Echium Oil with Oxidative Stability Increased by Emulsion Preparation in the Presence of the Phenolic Compound Sinapic Acid Followed by Dehydration by Spray and Freeze Drying Processes. J. Food Sci. Technol. 2019, 56, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Alhelli, A.M.; Hussin, A.S.M. Process Conditions of Spray Drying Microencapsulation of Nigella sativa Oil. Powder Technol. 2017, 315, 1–14. [Google Scholar] [CrossRef]
- Abedi, A.; Rismanchi, M.; Shahdoostkhany, M.; Mohammadi, A.; Hosseini, H. Microencapsulation of Nigella sativa Seeds Oil Containing Thymoquinone by Spray-drying for Functional Yogurt Production. Int. J. Food Sci. Technol. 2016, 51, 2280–2289. [Google Scholar] [CrossRef]
- Burhan, A.M.; Abdel-Hamid, S.M.; Soliman, M.E.; Sammour, O.A. Optimisation of the Microencapsulation of Lavender Oil by Spray Drying. J. Microencapsul. 2019, 36, 250–266. [Google Scholar] [CrossRef]
- Yekdane, N.; Goli, S.A.H. Effect of Pomegranate Juice on Characteristics and Oxidative Stability of Microencapsulated Pomegranate Seed Oil Using Spray Drying. Food Bioprocess. Technol. 2019, 12, 1614–1625. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Encapsulation of Carotenoid-Rich Oil from Gac Peel: Optimisation of the Encapsulating Process Using a Spray Drier and the Storage Stability of Encapsulated Powder. Powder Technol. 2019, 344, 373–379. [Google Scholar] [CrossRef]
- Chang, H.W.; Tan, T.B.; Tan, P.Y.; Nehdi, I.A.; Sbihi, H.M.; Tan, C.P. Microencapsulation of Fish Oil-in-Water Emulsion Using Thiol-Modified β-Lactoglobulin Fibrils-Chitosan Complex. J. Food Eng. 2020, 264, 109680. [Google Scholar] [CrossRef]
- Linke, A.; Weiss, J.; Kohlus, R. Oxidation Rate of the Non-Encapsulated-and Encapsulated Oil and Their Contribution to the Overall Oxidation of Microencapsulated Fish Oil Particles. Food Res. Int. 2020, 127, 108705. [Google Scholar] [CrossRef]
- Murali, S.; Kar, A.; Patel, A.S.; Mohapatra, D.; Krishnakumar, P. Optimization of Rice Bran Oil Encapsulation Using Jackfruit Seed Starch–Whey Protein Isolate Blend as Wall Material and Its Characterization. Int. J. Food Eng. 2017, 13, 4. [Google Scholar] [CrossRef]
- Yingngam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A. Response Surface Optimization of Spray-Dried Citronella Oil Microcapsules with Reduced Volatility and Irritation for Cosmetic Textile Uses. Powder Technol. 2019, 355, 372–385. [Google Scholar] [CrossRef]
- De Barros Fernandes, R.V.; Botrel, D.A.; Silva, E.K.; Pereira, C.G.; Do Carmo, E.L.; De Abreu Dessimoni, A.L.; Borges, S.V. Microencapsulated Ginger Oil Properties: Influence of Operating Parameters. Dry. Technol. 2017, 35, 1098–1107. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour Encapsulation and Controlled Release—A Review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Turchiuli, C.; Lemarié, N.; Cuvelier, M.-E.; Dumoulin, E. Production of Fine Emulsions at Pilot Scale for Oil Compounds Encapsulation. J. Food Eng. 2013, 115, 452–458. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Augustin, M.A.; Sanguansri, L. Encapsulation of Bioactives. In Food Materials Science; Springer: Berlin/Heidelberg, Germany, 2008; pp. 577–601. [Google Scholar]
- Verdalet-Guzmán, I.; Martínez-Ortiz, L.; Martínez-Bustos, F. Characterization of New Sources of Derivative Starches as Wall Materials of Essential Oil by Spray Drying. Food Sci. Technol. 2013, 33, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yuan, Y.; Yue, T. The Application of Starch-based Ingredients in Flavor Encapsulation. Starch Stärke 2015, 67, 225–236. [Google Scholar] [CrossRef]
- Sanchez, V.; Baeza, R.; Galmarini, M.V.; Zamora, M.C.; Chirife, J. Freeze-Drying Encapsulation of Red Wine Polyphenols in an Amorphous Matrix of Maltodextrin. Food Bioprocess. Technol. 2013, 6, 1350–1354. [Google Scholar] [CrossRef]
- Hadnađev, M.; Hadnađev, T.D.; Torbica, A.; Dokić, L.; Pajin, B.; Krstonošić, V. Rheological Properties of Maltodextrin Based Fat-Reduced Confectionery Spread Systems. Procedia Food Sci. 2011, 1, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, E.M.; Mulvihill, D.M.; O’Mahony, J.A. Physicochemical Properties of Whey Protein Conjugated with Starch Hydrolysis Products of Different Dextrose Equivalent Values. Int. Dairy J. 2016, 53, 20–28. [Google Scholar] [CrossRef]
- Da Silva Carvalho, A.G.; da Costa Machado, M.T.; da Silva, V.M.; Sartoratto, A.; Rodrigues, R.A.F.; Hubinger, M.D. Physical Properties and Morphology of Spray Dried Microparticles Containing Anthocyanins of Jussara (Euterpe edulis Martius) Extract. Powder Technol. 2016, 294, 421–428. [Google Scholar] [CrossRef]
- Daoub, R.M.A.; Elmubarak, A.H.; Misran, M.; Hassan, E.A.; Osman, M.E. Characterization and Functional Properties of Some Natural Acacia Gums. J. Saudi Soc. Agric. Sci. 2018, 17, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Dauqan, E.; Abdullah, A. Utilization of Gum Arabic for Industries and Human Health. Am. J. Appl. Sci. 2013, 10, 1270–1279. [Google Scholar] [CrossRef]
- Dos Santos Vaucher, A.C.; Dias, P.C.M.; Coimbra, P.T.; Dos Santos, I.M.C.; Marreto, R.N.; Dellamora-Ortiz, G.M.; De Freitas, O.; Ramos, M.F.S. Microencapsulation of Fish Oil by Casein-Pectin Complexes and Gum Arabic Microparticles: Oxidative Stabilization. J. Microencapsul. 2019, 36, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of Refined Kenaf (Hibiscus cannabinus, L.) Seed Oil by Spray Drying Using β-Cyclodextrin/Gum Arabic/Sodium Caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Emulsification and Microencapsulation Properties of Sodium Caseinate/Carbohydrate Blends. Int. Dairy J. 2001, 11, 137–144. [Google Scholar] [CrossRef]
- Fäldt, P.; Bergenståhl, B. Fat Encapsulation in Spray-Dried Food Powders. J. Am. Oil Chem. Soc. 1995, 72, 171–176. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of Wall Material Physicochemical Characteristics on the Stability of Encapsulated Phytochemicals: A Review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, K.; Zhong, Q. Eugenol Nanoencapsulated by Sodium Caseinate: Physical, Antimicrobial, and Biophysical Properties. Food Biophys. 2018, 13, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Ixtaina, V.Y.; Julio, L.M.; Wagner, J.R.; Nolasco, S.M.; Tomás, M.C. Physicochemical Characterization and Stability of Chia Oil Microencapsulated with Sodium Caseinate and Lactose by Spray-Drying. Powder Technol. 2015, 271, 26–34. [Google Scholar] [CrossRef]
- Shi, L.; Beamer, S.K.; Yang, H.; Jaczynski, J. Micro-Emulsification/Encapsulation of Krill Oil by Complex Coacervation with Krill Protein Isolated Using Isoelectric Solubilization/Precipitation. Food Chem. 2018, 244, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Domian, E.; Sułek, A.; Cenkier, J.; Kerschke, A. Influence of Agglomeration on Physical Characteristics and Oxidative Stability of Spray-Dried Oil Powder with Milk Protein and Trehalose Wall Material. J. Food Eng. 2014, 125, 34–43. [Google Scholar] [CrossRef]
- Rosida, D.F.; Mulyani, T.; Septalia, L.R. A Comparative Study of Non-Dairy Cream Based on the Type of Leguminosae Protein Source in Terms of Physico Chemical Properties and Organoleptic. Agric. Agric. Sci. Proced. 2016, 9, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, G.; Guida, L.; Martinez, V.; López, M.C.; Bernhardt, D.; Blasco, R.; Pedroza-Islas, R.; Hermida, L.G. Microencapsulation of Linseed Oil by Spray Drying for Functional Food Application. Food Res. Int. 2013, 52, 473–482. [Google Scholar] [CrossRef]
- Razmkhah, S.; Tan, C.; Long, K.; Nyam, K. Quality Changes and Antioxidant Properties of Microencapsulated Kenaf (Hibiscus cannabinus, L.) Seed Oil during Accelerated Storage. J. Am. Oil Chem. Soc. 2013, 90, 1859–1867. [Google Scholar] [CrossRef]
- Calvo, P.; Hernández, T.; Lozano, M.; González-Gómez, D. Microencapsulation of Extra-virgin Olive Oil by Spray-drying: Influence of Wall Material and Olive Quality. Eur. J. Lipid Sci. Technol. 2010, 112, 852–858. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Taneja, A.; Zhu, X.; Singh, H. Evaluation of Processed Cheese Fortified with Fish Oil Emulsion. Food Res. Int. 2009, 42, 1093–1098. [Google Scholar] [CrossRef]
- Aquilani, C.; Pérez-Palacios, T.; Sirtori, F.; Jiménez-Martín, E.; Antequera, T.; Franci, O.; Acciaioli, A.; Bozzi, R.; Pugliese, C. Enrichment of Cinta Senese Burgers with Omega-3 Fatty Acids. Effect of Type of Addition and Storage Conditions on Quality Characteristics. Grasas Aceites 2018, 69, 235. [Google Scholar] [CrossRef] [Green Version]
- Samsu, Z.; Zahir, A.Z.M. Production of Oil Palm Milk Powder by Spray Drying Technique. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Venturini, L.H.; Moreira, T.F.M.; da Silva, T.B.V.; de Almeida, M.M.C.; Francisco, C.R.L.; de Oliveira, A.; de Campos, S.S.; Bilck, A.P.; de Souza Leone, R.; Tanamati, A.A.C. Partial Substitution of Margarine by Microencapsulated Chia Seeds Oil in the Formulation of Cookies. Food Bioprocess. Technol. 2019, 12, 77–87. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Shojaee-Aliabadi, S.; Dadkhodazade, E.; Sheidaei, Z.; Abedi, A.-S.; Mirmoghtadaie, L.; Hosseini, S.M. Comparison of Properties of Breads Enriched with Omega-3 Oil Encapsulated in β-Glucan and Saccharomyces cerevisiae Yeast Cells. Appl. Food Biotechnol. 2019, 1, 11–20. [Google Scholar]
- Li, K.; Woo, M.W.; Patel, H.; Selomulya, C. Enhancing the Stability of Protein-Polysaccharides Emulsions via Maillard Reaction for Better Oil Encapsulation in Spray-Dried Powders by PH Adjustment. Food Hydrocoll. 2017, 69, 121–131. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S. Biscuits Fortified with Micro-Encapsulated Shrimp Oil: Characteristics and Storage Stability. J. Food Sci. Technol. 2017, 54, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Palacios, T.; Ruiz-Carrascal, J.; Jiménez-Martín, E.; Solomando, J.C.; Antequera, T. Improving the Lipid Profile of Ready-to-cook Meat Products by Addition of Omega-3 Microcapsules: Effect on Oxidation and Sensory Analysis. J. Sci. Food Agric. 2018, 98, 5302–5312. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-Grade Pickering Emulsion as a Novel Astaxanthin Encapsulation System for Making Powder-Based Products: Evaluation of Astaxanthin Stability during Processing, Storage, and Its Bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef] [PubMed]
- De Moura, S.C.S.R.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of Hibiscus Extract Encapsulated by Ionic Gelation Incorporated in Yogurt. Food Bioprocess. Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Bolger, Z.; Brunton, N.P.; Monahan, F.J. Impact of Inclusion of Flaxseed Oil (Pre-Emulsified or Encapsulated) on the Physical Characteristics of Chicken Sausages. J. Food Eng. 2018, 230, 39–48. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, M.Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Production of Functional Non-Dairy Creamer Using Nigella Sativa Oil via Fluidized Bed Coating Technology. Food Bioprocess. Technol. 2019, 8, 1352–1365. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Evaluating the Use of Fish Oil Microcapsules as Omega-3 Vehicle in Cooked and Dry-Cured Sausages as Affected by Their Processing, Storage and Cooking. Meat Sci. 2020, 162, 108031. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Agregán, R.; Lorenzo, J.M. Effect of the Partial Replacement of Pork Backfat by Microencapsulated Fish Oil or Mixed Fish and Olive Oil on the Quality of Frankfurter Type Sausage. J. Food Sci. Technol. 2017, 54, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of Healthier Oils to Enhance the Physicochemical and Nutritional Properties of Deer Pâté. LWT 2020, 125, 109223. [Google Scholar] [CrossRef]
- Heck, R.T.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Lorenzo, J.M.; Wagner, R.; Campagnol, P.C.B. Volatile Compounds and Sensory Profile of Burgers with 50% Fat Replacement by Microparticles of Chia Oil Enriched with Rosemary. Meat Sci. 2019, 148, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Stangierski, J.; Rezler, R.; Kawecki, K.; Peplińska, B. Effect of Microencapsulated Fish Oil Powder on Selected Quality Characteristics of Chicken Sausages. J. Sci. Food Agric. 2020, 100, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.G.; Jayathilakan, K.; Sarika, K.; Priya, E.R.; Greeshma, S.S.; Sultana, K.; Tejpal, C.S.; Mathew, S. Effect of Plectranthus Amboinicus Leaf Extract on the Quality Attributes of Microencapsulated Fish Oil Fortified Soup Powder; Society of Fisheries Technologists: Willingdon Island, India, 2019. [Google Scholar]
- Hastarini, E.; Napitupulu, R.J.; Poernomo, S.H. Characteristics of Instant Mushroom Cream Soup Enriched with Catfish Oil Microcapsules. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 12005. [Google Scholar]

| Core Material | Wall Material | Total Solids | Inlet/Outlet Temperature | Reference |
|---|---|---|---|---|
| Palm fibre oil | Gum Arabic | 20–40% | 130–202 °C/NM | [35] |
| Sour cherry oil | Maltodextrin + gum Arabic | 16.59–33.41% | 120–220 °C/NM | [36] |
| Walnut oil | SMP + Tween 80 | 30% | 180 °C/NM | [39] |
| Almond oil | Isolated starch | 30–40% | 145 °C/NM | [40] |
| Fish oil | Whey protein | 30% | 160 °C/NM | [41] |
| Corn oil | Brea gum | 30%–40% | 150 °C/60 °C | [42] |
| Virgin coconut oil | Soy protein isolate + maltodextrin | 20 to 30% | 160 and 180 °C | [43] |
| Palm Fibre Oil | Gum Arabic | 35% | 166 °C | [35] |
| PUFA-rich vegetable oil | Maltodextrin + modified starch | 2:1 (wall: oil) | 150 and 180 °C | [44] |
| Fish oil | Soybean protein | 1:1, 2:1, 3:1, 4:1 | 180 °C/96 °C | [2] |
| Rapeseed oil | Soy protein isolate + Maltodextrin | 30% | 140–220 °C/NM | [45] |
| Squash seed oil | Maltodextrin + gum Arabic | 25%,30%,35% | 140, 160, 180 °C/ 90 °C | [46] |
| Echium oil | Gum Arabic | 30% | 150 °C/NM | [47] |
| Nigella sativa oil | Maltodextrin + Sodium Caseinate | 20–60% | 150–190 °C/85 °C | [48] |
| Nigella sativa oil | Maltodextrin + gum Arabic | 30% | 160 °C/88 °C | [31] |
| Nigella sativa oil | Maltodextrin + sodium octenyl succinic starch | 25% | 140 °C/95 °C | [49] |
| Lavender oil | Maltodextrin + gum Arabic | 25%,30%,35% | 140 °C/95 °C | [50] |
| Pomegranate Seed Oil | Xanthan gum + gum Arabic | 30%,35%45% | 170 °C/85 °C | [51] |
| Gac peel oil | Whey protein + gum Arabic | 24.5% | 160 °C/NM | [52] |
| Fish oil | Chitosan + maltodextrin | 26.5% | 160, 170, 180 °C/NM | [53] |
| Fish oil | Soy protein isolate + maltodextrin | 45% | 160 °C/85 °C | [54] |
| Rice bran oil | Jackfruit seed starch + whey protein isolate | 30% | 140, 150 and 160 °C | [55] |
| Citronella oil | Gum Arabic | 20–60% | 136–203 °C | [56] |
| Ginger oil | Inulin + whey protein isolate | 20%,25%,30% | 140 °C,155 °C and 170 °C | [57] |
| Wall Material | Interest |
|---|---|
| Maltodextrin (DE < 20) | Film forming |
| Corn syrup solid (DE > 20) | Film forming, reducability |
| Modified starch | Very good emulsifier |
| Gum Arabic | Emulsifier, film forming |
| Modified cellulose | Film forming |
| Gelatin | Emulsifier, film forming |
| Cyclodextrin | Encapsulant, emulsifier |
| Lecithin | Emulsifier |
| Whey protein | Good emulsifier |
| Hydrogenated fat | Barrier to oxygen and water |
| Chitosan | Carrier of drug delivery |
| Encapsulated Oil | Product | Oil Source | Results | Reference |
|---|---|---|---|---|
| Fish oil | Burger | Marine | Burgers with microencapsulated fish oil showed the best scores for sensory traits and were stable during storage, and the thermal behaviour of the microparticles was similar before and after incorporation into the cookies. | [84] |
| Palm oil | Milk powder | Vegetable | The powders were easily soluble in water with low and non-hygroscopic moisture and low cohesiveness, which correspond to good flowability. | [85] |
| Chia oil | Cookies | Seed | Partial substitution of margarine by microencapsulated chia seed oil at 15 wt.% showed the best scores for sensory evaluation. | [86] |
| Flaxseed oil | Breads | Seed | Breads fortified with microencapsulated flaxseed oil showed lower peroxide index and higher α-linolenic acid value and helps preserve sensory properties compared to breads fortified with free flaxseed oil. | [87] |
| Canola oil | Non-dairy powder | Seed | Sodium caseinate and lactose via the Maillard reaction improved the encapsulation efficiency of oil up to 95.2%. | [88] |
| Shrimp oil | Biscuits | Marine | Biscuits fortified with 6% microencapsulated shrimp oil were stored in the dark to ensure their oxidative stability. | [89] |
| Fish oil | Chicken nuggets | Marine | Chicken nuggets enriched with microencapsulated fish oil showed no difference from control samples with respect to sensory attributes, and lower levels of lipid and protein oxidation were found microencapsulated fish oil. | [90] |
| Astaxanthin oil | Powder-based product | Marine | The encapsulation efficiency of astaxanthin powder was higher than 90%, the bioaccessebility of the reconstitution was around 80%, and it was stable under storage conditions. | [91] |
| Rapeseed oil | Yoghurt | Seed | Yoghurt matrix with microcapsules presented high acceptability of appearance and showed stability for 30 days. | [92] |
| Flaxseed oil | Chicken sausages | Seed | Spray-dried flaxseed oil formulations had lower values for cook loss and behaved differently during heating than the other formulations. | [93] |
| Nigella sativa oil | Non-dairy creamer | Seed | Microencapsulated oil demonstrated desired properties with high sensory acceptability for the revealed that developed non-dairy creamer. | [94] |
| Fish oil | Sausages | Marine | Fish oil microcapsules in cooked and dry-cured meat products labelled as “source of omega-3 fatty acids”, overall quality of the meat products enriched seems not to be impaired after storing. | [95] |
| Nigella sativa oil | Yoghurt | Seed | High stability of thymoquinone and proper chemical and sensory properties for yoghurt with Nigella sativa seeds oil microcapsules. | [49] |
| Fish oil | Sausage | Marine | The lipid oxidation increased, lipid reformulation increased MUFAs and n-3 PUFAs levels with highest TBARS values. | [96] |
| Tigernut, chia and linseed oils | Pâtés | Vegetable oils | Pâtés with microencapsulated oils showed modified fatty acid composition, decreasing the total amount of SFA and increasing PUFA (chia and linseed pâtés) or MUFA contents (tigernut pâtés). | [97] |
| Chia oil | Burgers | Seed | Microparticles of chia oil increased the terpenic volatiles and were characterized by the descriptors herbal and pleasant aroma and ideal texture with liking scores for sensory evaluation. | [98] |
| Fish oil | Chicken sausages | Marine | The sausages with the addition of microcapsules was characterized by higher values on the smell and consistency parameters with better results in the sensory evaluation. | [99] |
| Fish oil | Soup powder | Marine | The fortified soup powder of microencapsulated fish oil scored high in terms of sensory acceptance, proving its acceptability. | [100] |
| Catfish oil | Mushroom cream soup | Marine | Best physical characteristics of instant mushroom cream soup were reached with the addition of microcapsules at 3.6%. | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. https://doi.org/10.3390/molecules25173873
Mohammed NK, Tan CP, Manap YA, Muhialdin BJ, Hussin ASM. Spray Drying for the Encapsulation of Oils—A Review. Molecules. 2020; 25(17):3873. https://doi.org/10.3390/molecules25173873
Chicago/Turabian StyleMohammed, Nameer Khairullah, Chin Ping Tan, Yazid Abd Manap, Belal J. Muhialdin, and Anis Shobirin Meor Hussin. 2020. "Spray Drying for the Encapsulation of Oils—A Review" Molecules 25, no. 17: 3873. https://doi.org/10.3390/molecules25173873