Recovery of Lanthanum(III) and Nickel(II) Ions from Acidic Solutions by the Highly Effective Ion Exchanger
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Lewatit Monoplus SP112
2.2. Basic Parameters Effecting on Batch Sorption Experiments
2.2.1. Effect of HNO3 Concentration and Ion Exchanger Mass on Lanthanum(III) and Nickel(II) Sorption
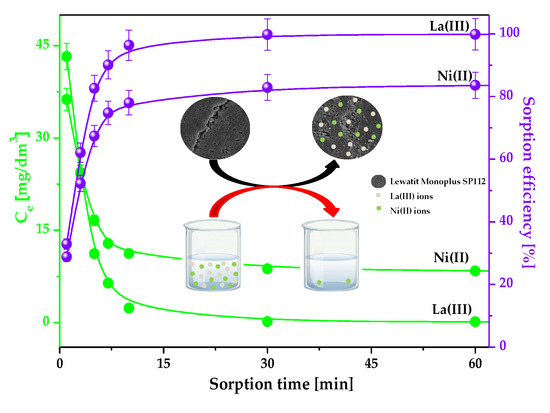
2.2.2. Effect of Phase Contact Time and Initial Metal Concentration on the Lanthanum(III) and Nickel(II) Sorption
2.3. Desorption Experiments
2.4. Kinetic Parameters for Lanthanum(III) and Nickel(II) Sorption
2.5. Equilibrium Isotherm and Thermodynamic Parameters for Lanthanum(III) and Nickel(II) Sorption
2.6. Column Experiment Results
2.7. La(III) Ions Sorption Studies in the Binary System
3. Conclusions
4. Materials and Methods
4.1. Materials in Experiments
4.2. Analytical Methods
4.3. Batch and Column Experiments
4.4. Calculations
4.4.1. Basic Parameters of the Batch Experiments
4.4.2. Basic Parameters of the Dynamic Experiments
4.4.3. Kinetic Parameters
- a)
- the Lagergren pseudo-first order (PFO):
- b)
- the Ho-McKay pseudo-second order (PSO) [27]:
- c)
- the intraparticle Weber-Morris diffusion (IPD):
- d)
- the Boyd model:
- e)
- the film diffusion model:
- f)
- the pore diffusion coefficient (Dp) [cm2/s]:
- g)
- the film diffusion coefficient (Df) [cm2/s]:
4.4.4. Isotherm Parameters
- a)
- the Langmuir isotherm model:
- b)
- the Freundlich isotherm model:
- c)
- the Temkin isotherm model:
4.4.5. Thermodynamic Parameters
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Z.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Yang, Y. A cleaner process for selective recovery of valuable metals from electronic waste of complex mixtures of end-of-life electronic products. Environ. Sci. Technol. 2015, 49, 7981–7988. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasir, S.M.; Hassan, S.S.M.; Kamel, A.H.; El-Nasr, R.S. Status of electronic waste recycling techniques: A review. Environ. Sci. Pollut. Res. 2018, 25, 16533–16547. [Google Scholar] [CrossRef] [PubMed]
- Balde, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor-2017; United Nations University (UNU): Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Vienna, Austria, 2017; ISBN ISBN 9789280845556. [Google Scholar]
- Zhang, S.; Huang, X.; Wang, D. Review on comprehensive recovery of valuable metals from spent electrode materials of nickel-hydrogen batteries. Rare Met. Mater. Eng. 2015, 44, 73–78. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Yang, M.; Cui, M.; Hou, X. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 2006, 84, 75–80. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Dominish, E.; Teske, S.; Florin, N. Responsible minerals sourcing for renewable energy. In Report Prepared for Earthworks by the Institute for Sustainable Futures; University of Technology Sydney: Ultimo, NSW, Australia, 2019. [Google Scholar]
- Sun, X.; Li, Z.; Wang, X.; Li, C. Technology development of electric vehicles: A review. Energies 2020, 13, 90. [Google Scholar] [CrossRef] [Green Version]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Lin, S.L.; Huang, K.L.; Wang, I.C.; Chou, I.C.; Kuo, Y.M.; Hung, C.H.; Lin, C. Characterization of spent nickel–metal hydride batteries and a preliminary economic evaluation of the recovery processes. J. Air Waste Manag. Assoc. 2016, 66, 296–306. [Google Scholar] [CrossRef]
- Porvali, A.; Ojanen, S.; Wilson, B.P.; Serna-Guerrero, R.; Lundström, M. Nickel metal hydride battery waste: Mechano-hydrometallurgical experimental study on recycling aspects. J. Sustain. Metall. 2020, 6, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Khalid, M.K.; Porvali, A.; Wilson, B.P.; Lundström, M. Recycling of spent NiMH batteries: Integration of battery leach solution into primary Ni production using solvent extraction. Sustain. Mater. Technol. 2019, 22, e00121. [Google Scholar] [CrossRef]
- Savov, G.; Angelov, T.; Tsekov, A.L.; Grigorova, I.; Nishkov, I. Combination of ion exchange and solvent extraction versus solvent extraction: A technical-economical comparison. In Proceedings of the 26th International Mineral Processing Congress, IMPC 2012: Innovative Processing for Sustainable Growth-Conference Proceedings, New Delphi, India, 24–28 September 2012; pp. 4779–4787. [Google Scholar]
- Bao, S.; Tang, Y.; Zhang, Y.; Liang, L. Recovery and Separation of Metal Ions from Aqueous Solutions by Solvent-Impregnated Resins. Chem. Eng. Technol. 2016, 39, 1377–1392. [Google Scholar] [CrossRef]
- Makanyire, T.; Sanchez-Segado, S.; Jha, A. Separation and recovery of critical metal ions using ionic liquids. Adv. Manuf. 2016, 4, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi, A.; Mahani Abolghasemi, A.; Izadi, A. Ion exchange resin technology in recovery of precious and noble metals. In Applications of Ion Exchange Materials in Chemical and Food Industries; Inamuddin Rangreez, T.A., Asiri, A.M., Eds.; Springer International Publishing: New York, NY, USA, 2019; pp. 193–258. ISBN 9783030060855. [Google Scholar]
- Otrembska, P.; Gega, J. Kinetic studies on sorption of Ni(II) and Cd(II) from chloride solutions using selected acidic cation exchangers. Physicochem. Probl. Miner. Process. 2013, 49, 301–312. [Google Scholar] [CrossRef]
- Lin, L.C.; Li, J.K.; Juang, R.S. Removal of Cu(II) and Ni(II) from aqueous solutions using batch and fixed-bed ion exchange processes. Desalination 2008, 225, 249–259. [Google Scholar] [CrossRef]
- Koopman, C.; Witkamp, G.J. Extraction of lanthanides from the phosphoric acid production process to gain a purified gypsum and a valuable lanthanide by-product. Hydrometallurgy 2000, 58, 51–60. [Google Scholar] [CrossRef]
- Khawassek, Y.M.; Eliwa, A.A.; Haggag, E.S.A.; Omar, S.A.; Abdel-Wahab, S.M. Adsorption of rare earth elements by strong acid cation exchange resin thermodynamics, characteristics and kinetics. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Bunkov, G.M.; Mashkovtsev, M.A.; Botalov, M.S.; Semenishchev, V.S.; Volkovich, V.A. Selective ion exchange recovery of rare earth elements from uranium mining solutions. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1767, p. 20017. [Google Scholar]
- Lokshin, E.P.; Tareeva, O.A.; Elizarova, I.R. Sorption of rare-earth elements from phosphogypsum sulfuric acid leaching solutions. Theor. Found. Chem. Eng. 2015, 49, 773–778. [Google Scholar] [CrossRef]
- Xu, J.; Virolainen, S.; Zhang, W.; Kuva, J.; Sainio, T.; Koivula, R. Polyacrylonitrile-encapsulated amorphous zirconium phosphate composite adsorbent for Co, Nd and Dy separations. Chem. Eng. J. 2018, 351, 832–840. [Google Scholar] [CrossRef]
- Virolainen, S.; Repo, E.; Sainio, T. Recovering rare earth elements from phosphogypsum using a resin-in-leach process: Selection of resin, leaching agent, and eluent. Hydrometallurgy 2019, 189, 105125. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Fila, D.; Hubicki, Z. Evaluation of possible use of the macroporous ion exchanger in the adsorption process of rare earth elements and heavy metal ions from spent batteries solutions. Chem. Eng. Process. Process Intensif. 2020, 147, 107767. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman characteristic group frequencies. In Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Lei, Y.; Cui, Y.; Huang, Q.; Dou, J.; Gan, D.; Deng, F.; Liu, M.; Li, X.; Zhang, X.; Wei, Y. Facile preparation of sulfonic groups functionalized Mxenes for efficient removal of methylene blue. Ceram. Int. 2019, 45, 17653–17661. [Google Scholar] [CrossRef]
- Brijmohan, S.B.; Swier, S.; Weiss, R.A.; Shaw, M.T. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B.; Barlas, H. Sorption of Ni(II) ions from aqueous solution by Lewatit cation-exchange resin. J. Hazard. Mater. 2009, 167, 915–926. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evaluation of chelate and cation exchange resins to remove copper ions. Powder Technol. 2016, 301, 520–525. [Google Scholar] [CrossRef]
- Guo, H.; Ren, Y.; Sun, X.; Xu, Y.; Li, X.; Zhang, T.; Kang, J.; Liu, D. Removal of Pb2+ from aqueous solutions by a high-efficiency resin. Appl. Surf. Sci. 2013, 283, 660–667. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. XPS study of sulfur and phosphorus compounds with different oxidation states. Sains Malaysiana 2018, 47, 1913–1922. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, Y.; Cong, B.; Xing, W.; Chen, G. Enhanced carriers separation efficiency in g-C3N4 modified with sulfonic groups for efficient photocatalytic Cr(VI) reduction. Mater. Res. Bull. 2020, 122, 110681. [Google Scholar] [CrossRef]
- Sarbak, Z. Adsorption and Adsorbents: Theory and Application; Polish Scientific Publishers UAM: Poznań, Poland, 2000. (In Polish) [Google Scholar]
- Ling, P.; Liu, F.; Li, L.; Jing, X.; Yin, B.; Chen, K.; Li, A. Adsorption of divalent heavy metal ions onto IDA-chelating resins: Simulation of physicochemical structures and elucidation of interaction mechanisms. Talanta 2010, 81, 424–432. [Google Scholar] [CrossRef]
- Kawamura, F.; Matsuda, M.; Aoyama, Y.; Chino, K.; Mizumoto, M. Method of Disposing Radioactive Ion Exchange Resin. U.S. Patent EP 0111 839 B1, 16 June 1987. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Alshahrie, A.; Darwesh, R.; Parveen, N.; Yadav, S.K.; Barakat, M.A.; Cho, M.H. Adsorption modeling and mechanistic insight of hazardous chromium on para toluene sulfonic acid immobilized-polyaniline@CNTs nanocomposites. J. Saudi Chem. Soc. 2019, 23, 188–197. [Google Scholar] [CrossRef]
- Michelsen, D.L.; Gideon, J.A.; Griffith, G.P.; Pace, J.E.; Kutat, H.L. Removal of soluble mercury from waste water by complexing techniques. In Virginia Water Resources Research Center Bulletin 74; Virginia Water Resources Research Center, Virginia Polytechnic Institute and State Univesity: Blacksburg, VA, USA, 1975. [Google Scholar]
- Zhou, F.; Feng, J.; Xie, X.; Wu, B.; Liu, Q.; Wu, X.; Chi, R. Adsorption of lanthanum(III) and yttrium(III) on kaolinite: Kinetics and adsorption isotherms. Physicochem. Probl. Miner. Process. 2019, 55, 928–939. [Google Scholar] [CrossRef]
- Kiruba, U.P.; Kumar, P.S.; Prabhakaran, C.; Aditya, V. Characteristics of thermodynamic, isotherm, kinetic, mechanism and design equations for the analysis of adsorption in Cd(II) ions-surface modified Eucalyptus seeds system. J. Taiwan Inst. Chem. Eng. 2014, 45, 2957–2968. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Bestani, B.; Benderdouche, N. The use of linear and nonlinear methods for adsorption isotherm optimization of basic green 4-dye onto sawdust-based activated carbon. J. Mater. Environ. Sci. 2018, 9, 1110–1118. [Google Scholar]
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Traisnel, M. Remediation of heavy metals by biomolecules: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1644–1704. [Google Scholar] [CrossRef]
- Côté, A.P.; Shimizu, G.K.H. The supramolecular chemistry of the sulfonate group in extended solids. Coord. Chem. Rev. 2003, 245, 49–64. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Y.; Wang, Q. Adsorption of lanthanum (III) from aqueous solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester-grafted magnetic silica nanocomposites. J. Hazard. Mater. 2013, 260, 409–419. [Google Scholar] [CrossRef]
- Kusrini, E.; Wicaksono, W.; Gunawan, C.; Daud, N.Z.A.; Usman, A. Kinetics, mechanism, and thermodynamics of lanthanum adsorption on pectin extracted from durian rind. J. Environ. Chem. Eng. 2018, 6, 6580–6588. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, S.; Zhao, K.; Wang, Z.; Xu, S.; Liang, Z.; Wu, K. Adsorption of La3+ and Ce3+ by poly-γ-glutamic acid crosslinked with polyvinyl alcohol. J. Rare Earths 2015, 33, 884–891. [Google Scholar] [CrossRef]
- Es-Sahbany, H.; Berradi, M.; Nkhili, S.; Hsissou, R.; Allaoui, M.; Loutfi, M.; Bassir, D.; Belfaquir, M.; El Youbi, M.S. Removal of heavy metals (nickel) contained in wastewater-models by the adsorption technique on natural clay. Mater. Today Proc. 2019, 13, 866–875. [Google Scholar] [CrossRef]
- Xu, M.; Liu, J.; Hu, K.; Xu, C.; Fang, Y. Nickel(II) removal from water using silica-based hybrid adsorbents: Fabrication and adsorption kinetics. Chin. J. Chem. Eng. 2016, 24, 1353–1359. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Modelling individual and competitive adsorption of cadmium(II) and zinc(II) metal ions from aqueous solution onto bagasse fly ash. Sep. Sci. Technol. 2006, 41, 2685–2710. [Google Scholar] [CrossRef]
- Girish, C.R. Multicomponent adsorption and the interaction between the adsorbent and the adsorbate: A review. Int. J. Mech. Eng. Technol. 2018, 9, 177–188. [Google Scholar]
- Dong, C.; Zhang, F.; Pang, Z.; Yang, G. Efficient and selective adsorption of multi-metal ions using sulfonated cellulose as adsorbent. Carbohydr. Polym. 2016, 151, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |














| Characteristics | Value |
|---|---|
| Matrix | Crosslinked polystyrene and divinylbenzene |
| Physical form | Spherical beige-gray beads |
| Functional groups |  sulfonic acid sulfonic acid
|
| Ionic form | Na+ |
| Total exchange capacity [val/dm3] | 1.7 |
| Average grain size [mm] | 0.65 |
| Bulk density [g/dm3] | 740 |
| Uniformity coefficient | 1.10 |
| pH operating range | 0–14 |
| Maximum temperature operating [K] | 393 |
| pHpzc | 6.61 |
| SBET [m2/g] | 14.98 |
| D [nm] | 32.72 |
| V [cm3/g] | 0.144 |
| Image |  |
| C0 [mg/dm3] | qexp [mg/g] | Pseudo-First Order | Pseudo-Second Order | ||||||||
| q1 [mg/g] | k1 [1/min] | R2 | q2 [mg/g] | k2 [g/mg·min] | R2 | ||||||
| La(III) | |||||||||||
| 25 | 3.13 | 0.47 | 0.055 | 0.949 | 3.14 | 0.496 | 0.999 | ||||
| 50 | 6.44 | 0.59 | 0.044 | 0.711 | 6.46 | 0.171 | 0.999 | ||||
| 100 | 12.41 | 1.00 | 0.049 | 0.769 | 12.45 | 0.121 | 0.999 | ||||
| 150 | 17.15 | 1.64 | 0.041 | 0.724 | 17.21 | 0.071 | 0.999 | ||||
| 200 | 23.24 | 2.36 | 0.046 | 0.803 | 23.31 | 0.054 | 0.999 | ||||
| Ni(II) | |||||||||||
| 25 | 2.35 | 0.36 | 0.023 | 0.679 | 2.36 | 0.346 | 1.000 | ||||
| 50 | 4.31 | 0.79 | 0.030 | 0.839 | 4.33 | 0.185 | 1.000 | ||||
| 100 | 9.27 | 1.44 | 0.026 | 0.756 | 9.30 | 0.097 | 1.000 | ||||
| 150 | 12.64 | 1.84 | 0.018 | 0.579 | 12.66 | 0.063 | 1.000 | ||||
| 200 | 16.56 | 3.33 | 0.018 | 0.662 | 16.61 | 0.032 | 0.999 | ||||
| C0 [mg/dm3] | Weber-Morris Intraparticle Diffusion | Boyd | |||||||||
| ki1* | C1 | R2 | ki2* | C2 | R2 | ki3* | C3 | R2 | Bt | R2 | |
| La(III) | |||||||||||
| 25 | 0.86 | 0.74 | 0.977 | 0.03 | 2.91 | 0.608 | 0.01 | 3.09 | 0.509 | 0.003 | 0.949 |
| 50 | 2.31 | 0.79 | 0.984 | 0.10 | 5.73 | 0.657 | 0.01 | 6.43 | 0.954 | 0.005 | 0.711 |
| 100 | 3.95 | 1.16 | 0.978 | 0.15 | 11.40 | 0.656 | 0.01 | 12.40 | 0.832 | 0.005 | 0.769 |
| 150 | 5.62 | 1.44 | 0.976 | 0.27 | 15.31 | 0.645 | 0.01 | 17.12 | 0.845 | 0.005 | 0.724 |
| 200 | 7.88 | 1.46 | 0.965 | 0.34 | 20.89 | 0.598 | 0.02 | 23.21 | 0.746 | 0.005 | 0.803 |
| Ni(II) | |||||||||||
| 25 | 0.76 | 0.08 | 0.993 | 0.04 | 2.02 | 0.807 | 0.01 | 2.30 | 0.986 | 0.003 | 0.679 |
| 50 | 1.45 | 0.13 | 0.989 | 0.08 | 3.67 | 0.848 | 0.01 | 4.23 | 0.816 | 0.003 | 0.839 |
| 100 | 2.93 | 0.74 | 0.991 | 0.17 | 7.98 | 0.850 | 0.01 | 9.09 | 0.891 | 0.003 | 0.757 |
| 150 | 3.46 | 2.06 | 0.970 | 0.24 | 10.77 | 0.622 | 0.02 | 12.25 | 0.958 | 0.003 | 0.579 |
| 200 | 4.17 | 2.45 | 0.950 | 0.33 | 13.78 | 0.724 | 0.05 | 15.70 | 0.952 | 0.002 | 0.663 |
| C0 [mg/dm3] | Film Diffusion Model | Film Diffusion Coefficient Df [cm2/s] | Pore Diffusion Coefficient Dp [cm2/s] | ||||||||
| kf [1/min] | R2 | ||||||||||
| La(III) | |||||||||||
| 25 | 11.43 | 0.949 | 2.04 × 10−7 | 1.76 × 10−8 | |||||||
| 50 | 9.54 | 0.711 | 1.72 × 10−6 | 1.76 × 10−8 | |||||||
| 100 | 9.38 | 0.769 | 6.12 × 10−6 | 1.76 × 10−8 | |||||||
| 150 | 8.77 | 0.724 | 6.40 × 10−6 | 8.80 × 10−9 | |||||||
| 200 | 8.73 | 0.803 | 9.12 × 10−6 | 8.80 × 10−9 | |||||||
| Ni(II) | |||||||||||
| 25 | 6.58 | 0.680 | 1.29 × 10−7 | 8.80 × 10−9 | |||||||
| 50 | 6.02 | 0.840 | 4.56 × 10−7 | 8.80 × 10−9 | |||||||
| 100 | 5.26 | 0.757 | 2.04 × 10−6 | 8.80 × 10−9 | |||||||
| 150 | 4.42 | 0.590 | 2.59 × 10−6 | 5.97 × 10−9 | |||||||
| 200 | 4.37 | 0.663 | 6.76 × 10−6 | 5.97 × 10−9 | |||||||
| T [K] | qexp [mg/g] | Langmuir | ||||||||
| qm [mg/g] | KL [dm3/mg] | R2 | Χ2 | RMSE | ||||||
| La(III) | ||||||||||
| 293 | 92.06 | 93.21 | 1.169 | 0.999 | 0.006 | 0.56 | ||||
| 313 | 92.90 | 94.61 | 1.192 | 0.996 | 0.009 | 0.67 | ||||
| 333 | 93.74 | 95.34 | 1.702 | 0.998 | 0.014 | 0.81 | ||||
| Ni(II) | ||||||||||
| 293 | 48.64 | 55.30 | 0.012 | 0.990 | 0.344 | 0.21 | ||||
| 313 | 49.60 | 59.17 | 0.018 | 0.991 | 0.073 | 1.33 | ||||
| 333 | 53.32 | 60.81 | 0.019 | 0.998 | 0.004 | 0.32 | ||||
| T [K] | Freundlich | Temkin | ||||||||
| KF [mg/g] | N | R2 | Χ2 | RMSE | A [dm3/g] | B [J/mol] | R2 | Χ2 | RMSE | |
| La(III) | ||||||||||
| 293 | 35.42 | 2.11 | 0.688 | 161.91 | 127.63 | 27.093 | 163.82 | 0.921 | 1.66 | 9.32 |
| 313 | 37.73 | 2.22 | 0.594 | 104.55 | 95.99 | 31.621 | 161.02 | 0.904 | 1.54 | 8.99 |
| 333 | 44.44 | 2.08 | 0.699 | 150.14 | 121.91 | 43.038 | 163.41 | 0.896 | 0.98 | 7.15 |
| Ni(II) | ||||||||||
| 293 | 1.380 | 1.53 | 0.959 | 21.01 | 27.22 | 0.217 | 246.91 | 0.936 | 1.03 | 4.79 |
| 313 | 2.227 | 1.74 | 0.957 | 15.37 | 22.90 | 0.368 | 273.27 | 0.959 | 1.38 | 5.56 |
| 333 | 2.251 | 1.64 | 0.954 | 26.37 | 32.44 | 0.372 | 242.89 | 0.953 | 0.81 | 4.50 |
| Sorbents | Conditions | qe [mg/g] | Literature | ||
|---|---|---|---|---|---|
| pH | t [min] | T [K] | |||
| La(III) ions sorption | |||||
| Kaolinite | 5.0 | 360 | 323 | 2.75 | [41] |
| Magnetic silica nanocomposite | 5.5 | 30 | 298 | 55.90 | [46] |
| Pectin from durian rind | 4.0 | 90 | 298 | 41.20 | [47] |
| Poly-γ-glutamic acid crosslinked with polyvinyl alcohol | 6.0 | 60 | 303 | 8.99 | [48] |
| Lewatit Monoplus SP112 | 1.5 | 30 | 333 | 93.74 | This study |
| Ni(II) ions sorption | |||||
| Lewatit Monoplus SP112Amberlite 200CAmberlyst 15 | 1.0 1.0 1.0 | 30 30 30 | - - - | 33.73 34.20 29.57 | [18] |
| Natural clay | 5.5 | 120 | 298 | 6.25 | [49] |
| Silica-based hybrid adsorbent | - | 10 080 | 298 | 49.24 | [50] |
| LewatitMonoplus SP112 | 1.5 | 60 | 333 | 53.32 | This study |
| T [K] | Kc [dm3/g] | ΔH° [kJ/mol] | ΔS° [J/mol·K] | ΔG° [kJ/mol] |
|---|---|---|---|---|
| La(III) | ||||
| 293 | 58.34 | −26.73 | ||
| 313 | 75.96 | 15.41 | 76.40 | −29.24 |
| 333 | 108.59 | −32.10 | ||
| Ni(II) | ||||
| 293 | 0.55 | −15.38 | ||
| 313 | 1.09 | 12.54 | 48.40 | −18.21 |
| 333 | 1.17 | −19.55 | ||
| U [cm3] | [cm3] | qec [mg/g] | Ct [mg/cm3] | Cw [mg/cm3] | Dg | Dv | %D |
|---|---|---|---|---|---|---|---|
| La(III) | |||||||
| 9200 | 12050 | 49.12 | 53.77 | 41.05 | 1442.59 | 1204.56 | 99.96 |
| Ni(II) | |||||||
| 1800 | 2550 | 10.86 | 12.85 | 9.07 | 304.86 | 254.56 | 99.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodyńska, D.; Fila, D.; Hubicki, Z. Recovery of Lanthanum(III) and Nickel(II) Ions from Acidic Solutions by the Highly Effective Ion Exchanger. Molecules 2020, 25, 3718. https://doi.org/10.3390/molecules25163718
Kołodyńska D, Fila D, Hubicki Z. Recovery of Lanthanum(III) and Nickel(II) Ions from Acidic Solutions by the Highly Effective Ion Exchanger. Molecules. 2020; 25(16):3718. https://doi.org/10.3390/molecules25163718
Chicago/Turabian StyleKołodyńska, Dorota, Dominika Fila, and Zbigniew Hubicki. 2020. "Recovery of Lanthanum(III) and Nickel(II) Ions from Acidic Solutions by the Highly Effective Ion Exchanger" Molecules 25, no. 16: 3718. https://doi.org/10.3390/molecules25163718