Synergistic Effect of a Pleuromutilin Derivative with Tetracycline against Streptococcus suis In Vitro and in the Neutropenic Thigh Infection Model
Abstract
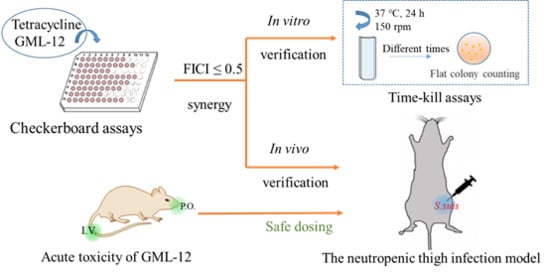
:1. Introduction
2. Results
2.1. In Vitro Antibacterial Activity
2.1.1. MIC and Checkerboard Assay
2.1.2. Time-Killing Assays of Synergistic Combinations
2.2. In Vivo Assessment
2.2.1. Acute Toxicity of GML-12 in Mice
2.2.2. In Vivo Synergistic Effects of GML-12/TET Combination
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms
4.3. Animal
4.4. In Vitro Antibacterial Activity
4.4.1. Determination of Minimum Inhibitory Concentration
4.4.2. Checkerboard Microdilution Assay
4.4.3. Time-Killing Assay
4.5. In Vivo Assessment
4.5.1. In Vivo Toxicity
4.5.2. In Vivo Anti-S. suis Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gottschalk, M.; Segura, M. Streptococcosis. Dis. Swine 2019, 61, 934–950. [Google Scholar]
- Rayanakorn, A.; Goh, B.H.; Lee, L.H.; Khan, T.M.; Saokaew, S. Risk factors for Streptococcus suis infection: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 13358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragojlovic, J.; Milošević, B.; Sasic, N.; Pelemis, M.; Sasic, M. Streptococcus suis infection: Clinical manifestations. Med. Pregl. 2005, 58, 236–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Wang, C.; Feng, Y.; Yang, W.; Song, H.; Chen, Z.; Yu, H.; Pan, X.; Zhou, X.; Wang, H.; et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. Ser. 2006, 3, e151. [Google Scholar]
- Yu, H.; Jing, H.; Chen, Z.; Zheng, H.; Zhu, X.; Wang, H.; Wang, S.; Liu, L.; Zu, R.; Luo, L.; et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 2006, 12, 914–920. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, H.; Wu, Z.; Wang, S.; Cao, M.; Hu, D.; Wang, C. Streptococcus suisinfection. Virulence 2014, 5, 477–497. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.; Grenier, D. Understanding the virulence of Streptococcus suis: A veterinary, medical, and economic challenge. Méd. Mal. Infect. 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Esgleas, M.; Dominguez-Punaro, M.D.L.C.; Li, Y.; Harel, J.; Dubreuil, J.D.; Gottschalk, M. Immunization with SsEno fails to protect mice against challenge with Streptococcus suisserotype 2. FEMS Microbiol. Lett. 2009, 294, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Pan, X.; Wen, S.; Wang, C.; Zhang, H.; Li, X.; Ying, M.; Shao, Z.; Ge, J.; Feng, Z. Streptococcus suis Enolase Functions as a protective antigen displayed on the bacterial cell surface. J. Infect. Dis. 2009, 200, 10. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.; Chung, P.H.; So, T.; Wong, L.; Choi, K.M.; Cheung, D.T.; Kam, K.M.; Chuang, S.K.; Tsang, T. On behalf of the collaborative study group on Streptococcus suis infection in Hong Kong Streptococcus suisinfection in Hong Kong: An emerging infectious disease? Epidemiol. Infect. 2008, 136, 1691–1697. [Google Scholar] [CrossRef]
- Salvarani, F.M.; Pinto, F.F.; Lobato, F.C.F.; Assis, R.A.D.; Costa, A.T.R. Mininum inhibitory concentration of eigth antibiotics toward isolates of Streptococcus suis. Braz. J. Vet. Res. Anim. Sci. 2008, 45, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Michalova, E.; Novotna, P.; Schlegelova, J. Tetracyclines in veterinary medicine and bacterial resistance to them. Veterinární Medicína 2012, 49, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Hernández-García, J.; Wang, J.; Restif, O.; Holmes, M.A.; Mather, A.E.; Weinert, L.A.; Wileman, T.; Thomson, J.R.; Langford, P.R.; Wren, B.W.; et al. Patterns of antimicrobial resistance in Streptococcus suis isolates from pigs with or without streptococcal disease in England between 2009 and 2014. Vet. Microbiol. 2017, 207, 117–124. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Wei, Z.; He, H.; Zhang, A.; Jin, M. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J. Vet. Med. Sci. 2012, 75, 583–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savic, M.; Årdal, C. A grant framework as a push incentive to stimulate research and development of new antibiotics. J. Law Med. Eth. A J. Am. Soc. Law Med. Eth. 2018, 46 (Suppl. 1), 9–24. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Sullivan, D.J. New uses for old drugs. Infect. Dis. Clin. N. Am. 2007, 448, 645–646. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yey, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xu, L.; Yuan, G.-J.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci. Rep. 2018, 8, 7237. [Google Scholar] [CrossRef]
- Siricilla, S.; Mitachi, K.; Yang, J.; Eslamimehr, S.; Lemieux, M.R.; Meibohm, B.; Ji, Y.; Kurosu, M. A new combination of a pleuromutilin derivative and doxycycline for treatment of multidrug-resistant acinetobacter baumannii. J. Med. Chem. 2017, 60, 2869–2878. [Google Scholar] [CrossRef] [Green Version]
- Schluenzen, F.; Pyetan, E.; Fucini, P.; Yonath, A.; Harms, J.M. Inhibition of peptide bond formation by pleuromutilins: The structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004, 54, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-L.; Zeng, J.; Fang, X.; Luo, J.; Jin, Z.; Liu, Y.-H.; Tang, Y.-Z. Design, synthesis and antibacterial evaluation of novel pleuromutilin derivatives possessing piperazine linker. Eur. J. Med. Chem. 2017, 127, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Giuliodori, A.M.; Spurio, R.; Milón, P.; Fabbretti, A. Antibiotics Targeting the 30S Ribosomal Subunit: A Lesson from Nature to Find and Develop New Drugs. Curr. Top. Med. Chem. 2019, 18, 2080–2096. [Google Scholar] [CrossRef] [PubMed]
- Callens, B.F.; Haesebrouck, F.; Maes, D.; Butaye, P.; Dewulf, J.; Boyen, F. Clinical resistance and decreased susceptibility in Streptococcus suis isolates from clinically healthy fattening pigs. Microb. Drug Resist. 2013, 19, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Gómez-Gascón, L.; Astorga, R.; Maldonado, A.; Huerta, B.; De Aguiar, F.C. Combined effect of conventional antimicrobials with essential oils and their main components against resistant Streptococcus suis strains. Lett. Appl. Microbiol. 2019, 68, 562–572. [Google Scholar] [CrossRef]
- Seitz, M.; Valentin-Weigand, P.; Willenborg, J. Use of antibiotics and antimicrobial resistance in veterinary medicine as exemplified by the swine pathogen Streptococcus suis. Curr. Top. Microbiol. Immunol. 2016, 398, 103–121. [Google Scholar] [CrossRef]
- Wongsawan, K.; Chaisri, W.; Tangtrongsup, S.; Mektrirat, R. Bactericidal effect of clove oil against multidrug-resistant Streptococcus suis isolated from human patients and slaughtered pigs. Pathogens 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Holmer, I.; Salomonsen, C.M.; Jorsal, S.E.; Astrup, L.B.; Jensen, V.F.; Høg, B.B.; Pedersen, K. Antibiotic resistance in porcine pathogenic bacteria and relation to antibiotic usage. BMC Vet. Res. 2019, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Petersen, P.J.; Labthavikul, P.; Jones, C.H.; Bradford, P.A. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J. Antimicrob. Chemother. 2006, 57, 573–576. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, J.-T.; Zheng, M.; Zhang, Q.; Walsh, T.R.; Liao, X.-P.; Sun, J.; Liu, Y.-H. Combination therapy strategies against multiple-resistant Streptococcus Suis. Front. Pharmacol. 2018, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Lorian, V. Differences between in vitro and in vivo studies. Antimicrob. Agents Chemother. 1988, 32, 1600–1601. [Google Scholar] [CrossRef] [Green Version]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-antimicrobial synergy: A medical and food perspective. Front. Microbiol. 2017, 8, 1205–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Performance Standards for Antimicrobial Susceptibility Testing CLSI M100-S25; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2015.
- Lebel, G.; Piché, F.; Frenette, M.; Gottschalk, M.; Grenier, D. Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides 2013, 50, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Aleinein, R.A.; Schäfer, H.; Wink, M. Secretory ranalexin produced in recombinant Pichia pastoris exhibits additive or synergistic bactericidal activity when used in combination with polymyxin B or linezolid against multi-drug resistant bacteria. Biotechnol. J. 2014, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, X.; Zhao, X.; Meng, R.; Liu, Z.; Chen, X.; Guo, N. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control 2017, 71, 10–16. [Google Scholar] [CrossRef]
- Ruppen, C.; Lupo, A.; Decosterd, L.A.; Sendi, P. Is penicillin plus gentamicin synergistic against clinical group B Streptococcus isolates? An In vitro Study. Front. Microbiol. 2016, 7, 1680–1689. [Google Scholar] [CrossRef]
- Zuo, G.-Y.; Fu, R.-C.; Yu, W.; Zhang, Y.-L.; Wang, G.-C. Potentiation effects by usnic acid in combination with antibiotics on clinical multi-drug resistant isolates of methicillin-resistant Staphylococcus aureus (MRSA). Med. Chem. Res. 2018, 27, 1443–1448. [Google Scholar] [CrossRef]
- Jindal, H.M.; Zandi, K.; Ong, K.C. Mechanisms of action and in vivo antibacterial efficacy assessment of five novel hybrid peptides derived from Indolicidin and Ranalexin against Streptococcus pneumoniae. PeerJ 2017, 5, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Lepak, A.J.; Marchillo, K.; VanHecker, J.; Andes, D.R. In vivo pharmacodynamic target assessment of eravacycline against escherichia coli in a murine thigh infection model. Antimicrob. Agents Chemother. 2017, 61, e00250-17. [Google Scholar] [CrossRef] [Green Version]
- Michail, G.; Labrou, M.; Pitiriga, V.; Manousaka, S.; Sakellaridis, N.; Tsakris, A.; Pournaras, S. Activity of tigecycline in combination with colistin, meropenem, rifampin, or gentamicin against KPC-producing enterobacteriaceae in a murine thigh infection model. Antimicrob. Agents Chemother. 2013, 57, 6028–6033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessier, P.R.; Nicolau, D.P. Tigecycline displaysin vivo bactericidal activity against extended-spectrum-β-lactamase-producing enterobacteriaceae after 72-Hour exposure period. Antimicrob. Agents Chemother. 2012, 57, 640–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |



| Strains | MIC (μg/mL) Along | MIC (μg/mL) Combined | ||||
|---|---|---|---|---|---|---|
| TET (Clinical Breakpoints a) | GML-12 | TET | GML-12 | FICI b | Interpretation | |
| ATCC 43765 | 1 (I) | 0.125 | 0.25 | 0.03125 | 0.5 | synergistic |
| S2013-1025 | 64 (R) | 8 | 32 | 4 | 1 | additivity |
| S94 | 64 (R) | 0.125 | 32 | 0.0625 | 1 | additivity |
| S41 | 128 (R) | 4 | 32 | 2 | 0.75 | additivity |
| S110 | 32 (R) | 0.25 | 4 | 0.125 | 0.625 | additivity |
| S114 | 64 (R) | 0.125 | 8 | 0.0625 | 0.625 | additivity |
| S40 | 64 (R) | 0.25 | 16 | 0.0625 | 0.5 | synergistic |
| S1-2 | 0.25 (S) | 0.0625 | 0.0625 | 0.03125 | 0.75 | additivity |
| S11 | 128 (R) | 4 | 32 | 1 | 0.5 | synergistic |
| S12 | 64 (R) | 0.25 | 16 | 0.125 | 0.75 | additivity |
| S75 | 64 (R) | 0.25 | 32 | 0.125 | 1 | additivity |
| SNJ-5 | 64 (R) | 8 | 8 | 2 | 0.375 | synergistic |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wei, M.-C.; Luo, Y.-D.; Jin, Z.; Tang, Y.-Z. Synergistic Effect of a Pleuromutilin Derivative with Tetracycline against Streptococcus suis In Vitro and in the Neutropenic Thigh Infection Model. Molecules 2020, 25, 3522. https://doi.org/10.3390/molecules25153522
Chen F, Wei M-C, Luo Y-D, Jin Z, Tang Y-Z. Synergistic Effect of a Pleuromutilin Derivative with Tetracycline against Streptococcus suis In Vitro and in the Neutropenic Thigh Infection Model. Molecules. 2020; 25(15):3522. https://doi.org/10.3390/molecules25153522
Chicago/Turabian StyleChen, Fang, Meng-Chao Wei, Yi-Dan Luo, Zhen Jin, and You-Zhi Tang. 2020. "Synergistic Effect of a Pleuromutilin Derivative with Tetracycline against Streptococcus suis In Vitro and in the Neutropenic Thigh Infection Model" Molecules 25, no. 15: 3522. https://doi.org/10.3390/molecules25153522