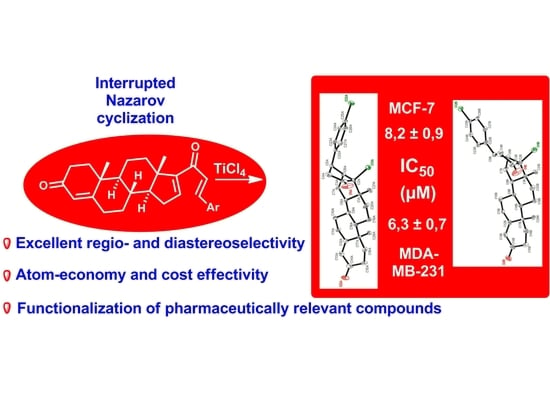
Novel d-Annulated Pentacyclic Steroids: Regioselective Synthesis and Biological Evaluation in Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Gram-Scale Synthesis of Pentacyclic Steroid 2g
2.3. X-ray Diffraction Study and Structure Optimization
2.4. In Vitro Antiproliferative Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization of Pentacyclic Steroids 2a–m
3.2.1. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-6a,8a-dimethyl-11-phenyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2a)
3.2.2. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-11-(4-chlorophenyl)-8b-chloro-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2b)
3.2.3. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-11-(4-bromophenyl)-8b-chloro-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2c)
3.2.4. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-11-(3-bromophenyl)-8b-chloro-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2d)
3.2.5. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-11-(2-fluorophenyl)-8b-chloro-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2e)
3.2.6. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(4-fluorophenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2f)
3.2.7. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(2,4-dichlorophenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2g)
3.2.8. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(2-chloro-6-fluorophenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2h)
3.2.9. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(4-methoxyphenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2i)
3.2.10. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(3-methoxyphenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2j)
3.2.11. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(3,4-dimethoxyphenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2k)
3.2.12. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-11-(3,4,5-trimethoxyphenyl)-6a,8a-dimethyl-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2l)
3.2.13. (6aR,6bS,8aS,8bS,11R,11aS,12aS,12bR)-8b-chloro-6a,8a-dimethyl-11-(thiophen-2-yl)-1,6,6a,6b,7,8,8a,8b,10,11,11a,12,12a,12b-tetradecahydropentaleno[2,1-a]phenanthrene-4,9(2H,5H)-dione (2m)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Ke, S. Recent Progress of novel steroid derivatives and their potential biological properties. Mini-Rev. Med. Chem. 2018, 18, 745–775. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Steroids: Partial synthesis in medicinal chemistry. Nat. Prod. Rep. 2010, 27, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Heasley, B. Chemical synthesis of the cardiotonic steroid glycosides and related natural products. Chem. Eur. J. 2012, 18, 3092–3120. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Carvalho, J.F.S.; Neves, M.A.C.; Silvestre, S.M.; Leitao, A.J.; Silva, M.M.C.; SáeMelo, M.L. Anticancer steroids: Linking natural and semi-synthetic compounds. Nat. Prod. Rep. 2013, 30, 324–374. [Google Scholar] [CrossRef] [PubMed]
- Shagufta, A.I.; Panda, G. Quest for steroidomimetics: Amino acids derived steroidal and nonsteroidal architectures. Eur. J. Med. Chem. 2017, 133, 139–151. [Google Scholar] [CrossRef]
- Khan, M.O.F.; Lee, H.J. Synthesis and pharmacology of anti-Inflammatory steroidal antedrugs. Chem. Rev. 2008, 108, 5131–5145. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.-K.; Ryoo, C.H.; Yang, H.W.; Shim, J.-Y.; Kang, M.G.; Lee, K.W.; Kang, H.I. Synthesis and bioactivities of steroid derivatives as antifungal agents. Tetrahedron 1998, 54, 15899–15914. [Google Scholar] [CrossRef]
- Sieghart, W.; Savic, M.M. International union of basic and clinical pharmacology. CVI: GABAA receptor subtype- and function-selective ligands: Key issues in translation to humans. Pharmacolog. Rev. 2018, 70, 836–878. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Li, Y.-F.; Wang, J.-W.; Yu, B.; Shi, Y.-K.; Liu, H.-M. Multicomponent assembly of novel antiproliferative steroidal dihydropyridinyl spirooxindoles. Steroids 2016, 109, 22–28. [Google Scholar] [CrossRef]
- Zorumski, C.F.; Mennerick, S.; Isenberg, K.E.; Covey, D.F. Potential clinical uses of neuroactive steroids. Curr. Opin. Investig. Drugs 2000, 1, 360–369. [Google Scholar]
- Blanco, M.-J.; La, D.; Coughlin, Q.; Newman, C.A.; Griffin, A.M.; Harrison, B.L.; Salituro, F.G. Breakthroughs in neuroactive steroid drug discovery. Bioorg. Med. Chem. Lett. 2018, 28, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nongthombam, G.S.; Boruah, R.C. Divergent synthesis of steroid analogs from steroidal β-formylenamides, conjugated enones and β-formylvinyl halides. Heterocycles 2019, 98, 19–62. [Google Scholar]
- Ibrahim-Ouali, M. Total synthesis of steroids and heterosteroids from BISTRO. Steroids 2015, 98, 9–28. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Romero, E.; Hamze, K. Stereoselective synthesis of pentacyclic steroids functionalized at C-11. Steroids 2012, 77, 1092–1100. [Google Scholar] [CrossRef]
- Yu, B.; Shi, X.-J.; Zheng, Y.-F.; Fang, Y.; Zhang, E.; Yu, D.-Q.; Liu, H.-M. A novel [1,2,4]triazolo[1,5-a] pyrimidine-based phenyl-linked steroid dimer: Synthesis and its cytotoxic activity. Eur. J. Med. Chem. 2013, 69, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Sun, X.N.; Shi, X.J.; Qi, P.P.; Fang, Y.; Zhang, E.; Yu, D.Q.; Liu, H.M. Stereoselective synthesis of novel antiproliferative steroidal (E, E) dienamides through a cascade aldol/cyclization process. Steroids 2013, 78, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, S.; Liu, H.M. Recent advances on the synthesis and antitumor evaluation of exonuclear heterosteroids. Chin. J. Org. Chem. 2017, 37, 1952–1962. [Google Scholar] [CrossRef]
- Bansal, R.; Achary, P.C. Man-made cytotoxic steroids: Exemplary agents for cancer therapy. Chem. Rev. 2014, 114, 6986–7005. [Google Scholar] [CrossRef]
- Singh, H.; Jindal, D.P.; Yadav, M.R.; Kumar, M. Heterosteroids and drug research. Progr. Med. Chem. 1991, 28, 233–300. [Google Scholar]
- Zhang, Z.; Giampa, G.M.; Draghici, C.; Huang, Q.; Brewer, M. Synthesis of demissidine by a ring fragmentation 1,3-dipolar cycloaddition approach. Org. Lett. 2013, 15, 2100–2103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, M.; Liu, X.; Yu, B. Synthesis of steroidal saponins bearing an aromatic E ring. Tetrahedron Lett. 2007, 48, 7323–7326. [Google Scholar] [CrossRef]
- Arthan, D.; Svasti, J.; Kittakoop, P.; Pittayakhachonwut, D.; Tanticharoen, M.; Thebtaranonth, Y. Antiviral isoflavonoid sulfate and steroidal glycosides from the fruits of Solanum torvum. Phytochemistry 2002, 59, 459–463. [Google Scholar] [CrossRef]
- Mewshaw, R.E.; Taylor, M.D.; Smith, A.B., III. Indole diterpene synthetic studies. 2. First-generation total synthesis of (-)-paspaline. J. Org. Chem. 1989, 54, 3449–3462. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shinonaga, H.; Shigemori, H.; Ymeyama, A.; Shoji, N.; Arihara, Sh. Xestobergsterol C, a new pentacyclic steroid from the okinawan marine sponge IRCINIA sp. and absolute stereochemistry of xestobergsterol A. J. Nat. Prod. 1995, 58, 312–318. [Google Scholar] [CrossRef]
- Tietze, L.F.; Modi, A. Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 2000, 20, 304–322. [Google Scholar] [CrossRef]
- Lambert, J.J.; Belelli, D.; Harney, S.C.; Peters, J.A.; Frenguelli, B.G. Modulation of native and recombinant GABAA receptors by endogenous and synthetic neuroactive steroids. Brain Res. Rev. 2001, 37, 68–80. [Google Scholar] [CrossRef]
- Jiang, X.; Manion, B.D.; Benz, A.; Rath, N.P.; Evers, A.S.; Zorumski, C.F.; Mennerick, S.; Covey, D.F. Neurosteroid analogues. 9. Conformationally constrained pregnanes: Structure-activity studies of 13,24-cyclo-18,21-dinorcholane analogues of the GABA modulatory and anesthetic steroids (3r,5r)- and (3r,5â)-3-hydroxypregnan-20-one. J. Med. Chem. 2003, 46, 5334–5348. [Google Scholar] [CrossRef]
- Kamernitzky, A.V.; Levina, I.S. Pregna-D’-pentaranes, Progestins and Antiprogestins: I. Separation of Biological Functions of Steroid Hormones. Rus. J. Bioorg. Chem. 2005, 31, 105–118. [Google Scholar] [CrossRef]
- Kamernitzky, A.V.; Levina, I.S. Pregna-D’-pentaranes, Progestins and Antiprogestins: II. 1 Pathways and Realization Mechanisms of Separate Biological Functions of Steroid Hormones. Rus. J. Bioorg. Chem. 2005, 31, 199–209. [Google Scholar] [CrossRef]
- Shchelkunova, T.A.; Rubtsov, P.M.; Levina, I.S.; Kamerntsky, A.V.; Smirnov, A.N. Pregna-D-pentarane structure influences progesterone receptor affinity for DNA. Steroids 2002, 67, 323–332. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Shin, K.; Takeda, K.; Arihara, S.; Kobayashi, J.; Takei, M. Two unique pentacyclic steroids with cis C/D ring junction from Xestospongia bergquistia Fromont, powerful inhibitors of histamine release. J. Org. Chem. 1992, 11, 2996–2997. [Google Scholar] [CrossRef]
- Singh, R.; Panda, G. An overview of synthetic approaches for heterocyclic steroids. Tetrahedron 2013, 69, 2853–2884. [Google Scholar] [CrossRef]
- Baranovskii, A.V.; Litvinovskaya, R.P.; Khripach, V.A. Steroids with a side chain containing a heterocyclic fragment: Synthesis and transformations. Russ. Chem. Rev. 1993, 62, 661–682. [Google Scholar] [CrossRef]
- Monier, M.; El-Mekabaty, A.; Abdel-Latif, D.; DogruMert, B.; Elattar, K.M. Heterocyclic steroids: Efficient routes for annulation of pentacyclic steroidal pyrimidines. Steroids 2020, 154, 108548–108549. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Thota, S.; Bansal, R. Studies on 16,17-pyrazoline substituted heterosteroids as anti-Alzheimer and anti-Parkinsonian agents using LPS induced neuroinflammation models of mice and rats. Acs Chem. Neurosci. 2018, 9, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Komkov, A.V.; Komendantova, A.S.; Yastrebova, M.A.; Andreeva, O.E.; Shirinian, V.Z.; Hajra, A.; Zavarzin, I.V.; Volkova, Y.A. Steroidal pyrimidines and dihydrotriazines as novel classes of anticancer agents against hormone-dependent breast cancer cells. Front. Pharm. 2018, 8, 979–992. [Google Scholar] [CrossRef] [Green Version]
- Komendantova, A.S.; Scherbakov, A.M.; Komkov, A.V.; Chertkova, V.V.; Gudovanniy, A.O.; Chernoburova, E.I.; Sorokin, D.V.; Dzichenka, Ya.U.; Shirinian, V.Z.; Volkova, Yu.A.; et al. Novel steroidal 1,3,4-thiadiazines: Synthesis and biological evaluation in androgen receptor-positive prostate cancer 22Rv1 cells. Bioorg. Chem. 2019, 91, 103142. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Song, L.-X.; Li, Y.-F.; Li, Y.-L.; Guo, Y-Z.; Zhang, E.; Liu, H.-M. Synthesis and biological evaluation of dehydroepiandrosterone-fused thiazole, imidazo[2,1-b]thiazole, pyridine steroidal analogues. Steroids 2014, 80, 92–101. [Google Scholar] [CrossRef]
- Pilgrim, B.S.; Gatland, A.E.; Esteves, C.H.A.; McTernan, C.T.; Jones, G.R.; Tatton, M.R.; Procopiou, P.A.; Donohoe, T.J. Palladium-catalyzed enolatearylation as a key C–C bond-forming reaction for the synthesis of isoquinolines. Org. Biomol. Chem. 2016, 14, 1065–1090. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim-Ouali, M. Synthesis of pentacyclic steroids. Steroids 2008, 73, 775–797. [Google Scholar] [CrossRef]
- Zavarzin, I.V.; Chertkova, V.V.; Levina, I.S.; Chernoburova, E.I. Steroids fused to heterocycles at positions 16, 17 of the D-ring. Russ. Chem. Rev. 2011, 80, 661–682. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Gomes, C.S.B.; Pinho e Melό, T.M.V.D. Reactivity of steroidal 1-azadienes toward carbonyl Compounds under Enamine Catalysis: Chiral Penta- and hexacyclic steroids. Org. Lett. 2018, 20, 4332–4336. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Johnson, T.W. Synthesis of 7-deoxyxestobergestrol A, a novel pentacyclic steroid of the Xestobergestrol class. J. Am. Chem. Soc. 1997, 119, 12412–12413. [Google Scholar] [CrossRef]
- Sharma, U.; Ahmed, Sh.; Boruah, R.C. A facile synthesis of annelated pyridines from β-formyl enamides under microwave irradiation. Tetrahedron Lett. 2000, 41, 3493–3495. [Google Scholar] [CrossRef]
- Dutta, M.; Saikia, P.; Gogoi, S.; Boruah, R.C. Microwave-promoted and Lewis acid catalysed synthesis of steroidal A- and D-ring fused 4,6-diarylpyridines. Steroids 2013, 78, 387–395. [Google Scholar] [CrossRef]
- Schomburg, D.; Thielmann, M.; Winterfeldt, E. Dienes as chiral templates. Tetrahedron Lett. 1986, 27, 5833–5834. [Google Scholar] [CrossRef]
- LeBideau, F.; Dagorne, S. Synthesis of transition-metal steroid derivatives. Chem. Rev. 2013, 113, 7793–7850. [Google Scholar] [CrossRef]
- Gupta, A.; Sathish, B.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef]
- Krafft, M.E.; Dasse, O.A.; Fu, Z. Synthesis of the C/D/E and A/B rings of Xestobergsterol-(A). J. Org. Chem. 1999, 64, 2475–2485. [Google Scholar] [CrossRef]
- Chowdhury, P.; Borah, J.M.; Bordoloi, M.; Goswami, P.K.; Goswami, A.; Barua, N.C.; Rao, P.G. A simple efficient process for the synthesis of 16-dehydropregnenolone acetate (16-DPA)—A key steroid drug intermediate from diosgenin. J. Chem. Eng. Process Technol. 2011, 2, 117–124. [Google Scholar] [CrossRef]
- Kumar, M.; Rawat, P.; Khan, M.; Rawat, A.K.; Srivastava, A.K.; Maurya, R. Aza-annulation on the 16-dehydropregnenolone, via tandem intermolecular Aldol process and intramolecular Michael addition. Bioorganic Med. Chem. Lett. 2011, 21, 2232–2237. [Google Scholar] [CrossRef] [PubMed]
- Volkova, Yu.A.; Kozlov, A.S.; Kolokolova, M.K.; Uvarov, D.Y.; Gorbatov, S.A.; Andreeva, O.E.; Scherbakov, A.M.; Zavarzin, I.V. Steroidal N-sulfonylimidates: Synthesis and biological evaluation in breast cancer cells. Eur. J. Med. Chem. 2019, 179, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Zavarzin, I.V.; Vorontsova, S.K.; Hajra, A.; Andreeva, O.E.; Yadykov, A.V.; Levina, I.S.; Volkova, Y.A.; Shirinian, V.Z. Synthesis and evaluation of the antiproliferative activity of benzylidenes of 16-dehydroprogesterone series. Steroids 2018, 138, 91–101. [Google Scholar] [CrossRef]
- Yadykov, A.V.; Shirinian, V.Z. Recent advances in the interrupted Nazarov reaction. Adv. Synth. Catal. 2020, 4, 702–723. [Google Scholar] [CrossRef]
- Grant, T.N.; Riedera, C.J.; West, F.G. Interrupting the Nazarov reaction: Domino and cascade processes utilizing cyclopentenyl cations. Chem. Commun. 2009, 38, 5676–5688. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in the Nazarov process. Tetrahedron 2005, 61, 6479–6517. [Google Scholar] [CrossRef]
- Frontier, A.J.; Collison, C. The Nazarov cyclization in organic synthesis. Recent Adv. Tetrahedron 2005, 61, 7577–7606. [Google Scholar] [CrossRef]
- Vaidya, T.; Eisenberg, R.; Frontier, A.J. Catalytic Nazarov cyclization: The state of the art. Chem. Cat. Chem. 2011, 3, 1531–1548. [Google Scholar] [CrossRef]
- Denmark, S.E.; Jones, T.K. Silicon-directed Nazarov cyclization. J. Am. Chem. Soc. 1982, 104, 2642–2645. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Belfield, A.J. Catalytic asymmetric Nazarov reactions promoted by chiral Lewis acid complexes. Org. Lett. 2003, 5, 5075–5078. [Google Scholar] [CrossRef]
- Janka, M.; He, A.; Frontier, A.J. Efficient catalysis of Nazarov cyclization using a cationic iridium complex possessing adjacent labile coordination sites. J. Am. Chem. Soc. 2004, 126, 6864–6865. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Trauner, D. Enantioselective Nazarov reactions through catalytic asymmetric proton transfer. J. Am. Chem. Soc. 2004, 126, 9544–9545. [Google Scholar] [CrossRef]
- Shirinian, V.Z.; Lvov, A.G.; Yadykov, A.V.; Yaminova, L.V.; Kachala, V.V.; Markosyan, A.I. Triaryl-substituted divinyl ketones cyclization: Nazarov reaction versus Friedel–Crafts electrophilic substitution. Org. Lett. 2016, 18, 6260–6263. [Google Scholar] [CrossRef]
- Janka, M.; He, W.; Haedicke, I.E.; Fronczek, F.R.; Frontier, A.J.; Eisenberg, R. Tandem Nazarov cyclization−Michael addition sequence catalyzed by an Ir(III) complex. J. Am. Chem. Soc. 2006, 128, 5312–5313. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.J.; Winberg, K.J.; West, F.G. Cyclization of cross-conjugated trienes: The vinylogous Nazarov reaction. J. Am. Chem. Soc. 2009, 131, 7504–7505. [Google Scholar] [CrossRef] [PubMed]
- Dhoro, F.; Tius, M.A. Interrupted Nazarov cyclization on silica gel. J. Am. Chem. Soc. 2005, 127, 12472–12473. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Kawatsura, M.; Hayase, S.; Nanjo, M.; Itoh, T. Iron(III) Salt-catalyzed Nazarov cyclization/Michael addition of pyrrole derivatives. Adv. Synth. Catal. 2009, 351, 123–128. [Google Scholar] [CrossRef]
- Basak, A.K.; Shimada, N.; Bow, W.F.; Vicic, D.A.; Tius, M.A. An Organocatalytic asymmetric Nazarov cyclization. J. Am. Chem. Soc. 2010, 132, 8266–8267. [Google Scholar] [CrossRef] [Green Version]
- Murugan, K.; Srimurugan, S.; Chen, C.P. A mild, catalytic and efficient Nazarov cyclization mediated by phosphomolybdic acid. Chem. Commun. 2010, 46, 1127–1129. [Google Scholar] [CrossRef]
- Shirinian, V.Z.; Lvov, A.G.; Yanina, A.M.; Kachala, V.V.; Krayushkin, M. Synthesis of new photochromic diarylethenes of cyclopentenone series by Nazarov reaction. Chem. Heterocycl. Comp. 2015, 51, 234–241. [Google Scholar] [CrossRef]
- Vaidya, T.; Manbeck, G.F.; Chen, S.; Frontier, A.J.; Eisenberg, R. Divergent reaction pathways of a cationic intermediate: Rearrangement and cyclization of 2-substituted furyl and benzofurylenones catalyzed by iridium(III). J. Am. Chem. Soc. 2011, 133, 3300–3303. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Leboeuf, D.; Frontier, A.J. Understanding the fate of the oxyallyl cation following Nazarov electrocyclization: Aequential Wagner−Meerwein migrations and the synthesis of spirocycliccyclopentenones. J. Am. Chem. Soc. 2011, 133, 6307–6317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, J.A.; Arif, A.M.; West, F.G. Nazarov-initiated diastereoselective cascade polycyclization of aryltrienones. J. Am. Chem. Soc. 1999, 121, 7443–7444. [Google Scholar] [CrossRef]
- Saxena, H.O.; Faridi, U.; Kumar, J.K.; Luqman, S.; Darokar, M.P.; Shanker, K.; Chanotiya, C.S.; Gupta, M.M.; Negi, A.S. Synthesis of chalcone derivatives on steroidal framework and their anticancer activities. Steroids 2007, 72, 892–900. [Google Scholar] [CrossRef]
- Singh, R.; Panda, G. Application of Nazarov type electrocyclization to access [6,5,6] and [6,5,5] core embedded new polycycles: An easy entry to tetrahydrofluorene scaffolds related to Taiwaniaquinoids and C-nor-D homosteroids. Org. Biomol. Chem. 2011, 9, 4782–4790. [Google Scholar] [CrossRef]
- Mousavizadeh, F.; Meyer, D.; Giannis, A. Synthesis of C-nor-D-homo-steroidal alkaloids and their derivatives. Synthesis 2018, 50, 1587–1600. [Google Scholar]
- Krieger, J.; Smeilus, T.; Schackow, O.; Giannis, A. Lewis Acid mediated Nazarov cyclization as a convergent and enantioselective entry to C-nor-D-homo-Steroids. Chem. Eur. J. 2017, 23, 5000–5004. [Google Scholar] [CrossRef]
- Vada, E.; Fujiwara, I.; Kanemasa, S.; Tsuge, O. Synthesis of hexaindenones through a Diels-Alder cycloaddition and Nazarov cyclization sequence of triene alcohols. Bull. Chem. Soc. Jpn. 1987, 60, 325–334. [Google Scholar]
- He, W.; Sun, X.; Frontier, A.J. Polarizing the Nazarov cyclization: efficient catalysis under mild conditions. J. Am. Chem. Soc. 2003, 125, 14278–14279. [Google Scholar] [CrossRef]
- White, T.D.; West, F.G. Halide trapping of the Nazarov intermediate in strained polycyclic systems: A new interrupted Nazarov reaction. Tetrahedron Lett. 2005, 46, 5629–5632. [Google Scholar] [CrossRef]
- Marx, V.M.; Cameron, T.S.; Burnell, D.J. Formation of halogenated cyclopent-2-enone derivatives by interrupted Nazarov cyclizations. Tetrahedron Lett. 2009, 50, 7213–7216. [Google Scholar] [CrossRef]
- Schatz, D.J.; Kwon, Y.; Scully, T.W.; West, F.G. Interrupting the Nazarov cyclization with bromine. J. Org. Chem. 2016, 81, 12494–12498. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.E.; Coates, R.M. Stereoselective Prinscyclizations of δ,ε-unsaturated Ketones to cis-3-chlorocyclohexanols with TiCl4. Angew. Chem. Int. Ed. 2002, 41, 491–493. [Google Scholar] [CrossRef]
- Willmore, N.D.; Goodman, R.; Lee, H.-H.; Kennedy, R.M. A short synthesis of (.+-.)-.beta.-isocomene. J. Org. Chem. 1992, 57, 1216–1219. [Google Scholar] [CrossRef]
- Liu, H.-J.; Sun, D. Polyene cyclization promoted by the cross conjugated α-carbalkoxyenone system. Application to the total synthesis of (±)-Dehydrochamaecynenol. Tetrahedron Lett. 1997, 38, 6159–6162. [Google Scholar] [CrossRef]
- Browder, C.C.; West, F.G. Formation of hydrindans and tricyclo[4.3.0.03,8]nonanes via 6-endo trapping of the Nazarovoxyallyl intermediate. Synlett 1999, 1999, 1363–1366. [Google Scholar] [CrossRef]
- Harmata, M.; Elomari, S.; Barnes, C.L. Intramolecular 4 + 3 cycloadditions.Cycloaddition reactions of cyclic alkoxyallylic and oxyallylic cations. J. Am. Chem. Soc. 1996, 118, 2860–2871. [Google Scholar] [CrossRef]
- Crystallographic data was deposited with the Cambridge Crystallographic Data Centre (CCDC No. 1990621 and 1990622. For details, see section IV in the SM).
- Li, Z.; Boyd, R.J.; Burnell, D.J. Computational examination of (4 + 3) versus (3 + 2) Cycloaddition in the Interception of Nazarov reactions of allenyl vinyl ketones by dienes. J. Org. Chem. 2015, 80, 12535–12544. [Google Scholar] [CrossRef]
- Levenson, A.S.; Jordan, V.C. MCF-7: The first hormone-responsive breast cancer cell line. Cancer Res. 1997, 57, 3071–3078. [Google Scholar]
- Yu, K.; Rohr, J.; Liu, Y.; Li, M.; Xu, J.; Wang, K.; Chai, J.; Zhao, D.; Liu, Y.; Ma, J.; et al. Progress in triple negative breast carcinoma pathophysiology: Potential therapeutic targets. Pathol. Res. Pr. 2020, 216, 152874. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Zhang, Z.; Yang, Z. Steroidal[17,16-d]pyrimidines derived from dehydroepiandrosterone: A convenient synthesis, antiproliferation activity, structure-activity relationships, and role of heterocyclic moiety. Sci. Rep. 2017, 7, 44439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, M.M.M.; Amr, A.E.-G.E.; Abdalla, M.M.; Al-Omar, M.A.; Safwat, H.M.; Elgamal, M.H. Synthesis of androstanopyridine and pyrimidine compounds as novel activators of the tumor suppressor protein p53. Z. Nat. C 2015, 70, 205–216. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a–m are available from the authors. |







| Entry | Codes | Ar | Yields | |
|---|---|---|---|---|
| NMR * | Isolated | |||
| 1 | a | Ph | 80 | 63 |
| 2 | b | 4-Cl-C6H4 | 95 | 78 |
| 3 | c | 4-Br-C6H4 | 95 | 70 |
| 4 | d | 3-Br-C6H4 | 88 | 60 |
| 5 | e | 2-F-C6H4 | 71 | 40 |
| 6 | f | 4-F-C6H4 | 80 | 41 |
| 7 | g | 2,4-Cl2C6H3 | 92 | 73 |
| 8 | h | 2-Cl-6-F-C6H3 | 80 | 58 |
| 9 | i | 4-MeO-C6H4 | 78 | 55 |
| 10 | j | 3-MeO-C6H4 | 76 | 44 |
| 11 | k | 3,4-(MeO)2-C6H3 | 91 | 43 |
| 12 | l | 3,4,5-(MeO)3C6H2 | 70 | 47 |
| 13 | m | 2-Thienyl | 92 | 64 |
| 14 | n | 2-Furyl | 35 | 0 |
| IC50 a, µM | |||
|---|---|---|---|
| Entry | Compounds | MCF-7 | MDA-MB-231 |
| 1 | 2a | 10.0 ± 1.5 | 8.8 ± 0.9 |
| 2 | 2b | 6.0 ± 0.8 | 9.4 ± 1.1 |
| 3 | 2c | 8.1 ± 0.9 | >25 |
| 4 | 2d | 7.5± 0.9 | >25 |
| 5 | 2e | 12.6 ± 1.6 | 19.6 ± 2.1 |
| 6 | 2f | 7.1 ± 0.9 | 12.2 ± 1.6 |
| 7 | 2g | 8.2 ± 0.9 | 6.3 ± 0.7 |
| 8 | 2h | 17.7 ± 2.1 | 15.2 ± 1.6 |
| 9 | 2i | 9.5 ± 1.2 | 13.0 ± 1.5 |
| 10 | 2j | 7.2 ± 0.8 | >25 |
| 11 | 2k | 25 ± 2.2 | 24.8 ± 2.5 |
| 12 | 2l | 10.6 ± 1.3 | 21.5 ± 2.4 |
| 13 | 2m | 24.5 ± 2.5 | >25 |
| 14 | Cisplatin(CDDP) | 6.3 ± 0.8 | 13.2 ± 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorontsova, S.K.; Yadykov, A.V.; Scherbakov, A.M.; Minyaev, M.E.; Zavarzin, I.V.; Mikhaevich, E.I.; Volkova, Y.A.; Shirinian, V.Z. Novel d-Annulated Pentacyclic Steroids: Regioselective Synthesis and Biological Evaluation in Breast Cancer Cells. Molecules 2020, 25, 3499. https://doi.org/10.3390/molecules25153499
Vorontsova SK, Yadykov AV, Scherbakov AM, Minyaev ME, Zavarzin IV, Mikhaevich EI, Volkova YA, Shirinian VZ. Novel d-Annulated Pentacyclic Steroids: Regioselective Synthesis and Biological Evaluation in Breast Cancer Cells. Molecules. 2020; 25(15):3499. https://doi.org/10.3390/molecules25153499
Chicago/Turabian StyleVorontsova, Svetlana K., Anton V. Yadykov, Alexander M. Scherbakov, Mikhail E. Minyaev, Igor V. Zavarzin, Ekaterina I. Mikhaevich, Yulia A. Volkova, and Valerii Z. Shirinian. 2020. "Novel d-Annulated Pentacyclic Steroids: Regioselective Synthesis and Biological Evaluation in Breast Cancer Cells" Molecules 25, no. 15: 3499. https://doi.org/10.3390/molecules25153499