Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug−Resistant Foodborne Microorganisms
Abstract
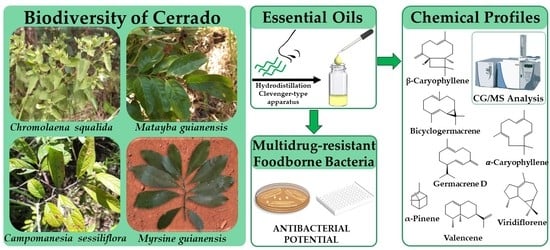
:1. Introduction
2. Results
2.1. Antimicrobial Screening of Cerrado Plants Essential Oils
2.2. Chemical Profile of the Bioactive Essential Oils
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization, Ed.; WHO: Geneva, Switzerland, 2015; ISBN 978-924-156-516-5. [Google Scholar]
- WHO. Strengthening Surveillance of and Response to Foodborne Diseases: A Practical Manual. Introductory Module; World Health Organization, Ed.; WHO: Geneva, Switzerland, 2017; ISBN 978-92-4-151324-1. [Google Scholar]
- Jaffee, S.; Henson, S.; Unnevehr, L.; Grace, D.; Cassou, E. The Safe. Food Imperative: Accelerating Progress in Low- and Middle-Income Countries. Agriculture and Food Series; World Bank: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- WHO. Whole Genome Sequencing for Foodborne Disease Surveillance: Landscape Paper; World Health Organization, Ed.; WHO: Geneva, Switzerland, 2018; ISBN 978-92-4-151386-9. [Google Scholar]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A Review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, S.; Huang, J.; Wu, Q.; Zhang, F.; Zhang, J.; Wang, J.; Ding, Y.; Zhang, S.; Yang, X.; et al. Prevalence and characterization of Staphylococcus aureus isolated from pasteurized milk in China. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; Tobin, C.; Ross, R.P.; Stanton, C.; Hill, C. Foodborne pathogens and zoonotic diseases. In Raw Milk: Balance between Hazards and Benefits, 1st ed.; Nero, L., De Carvalho, A.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 259–272. ISBN 978-0-12-810530-6. [Google Scholar]
- Rall, V.L.M.; Vieira, F.P.; Rall, R.; Vieitis, R.L.; Fernandes, A., Jr.; Candeias, J.M.G.; Cardoso, K.F.G.; Araújo, J.P., Jr. PCR detection of staphylococcal enterotoxin genes in Staphylococcus aureus strains isolated from raw and pasteurized milk. Vet. Microbiol. 2008, 132, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Beef. Available online: http://www.brazilianbeef.org.br/Summary.aspx (accessed on 9 May 2020).
- FAO, Food and Agriculture Organization of the United Nations. Dairy Market Review. March 2020. Available online: http://www.fao.org/dairy-production-products/resources/publications/fao-publications/en/ (accessed on 4 May 2020).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imane, N.I.; Fouzia, H.; Azzahra, L.F.; Ahmed, E.; Idrissa, D.; Mohamed, K.-H.; Sirine, F.; L’Houcine, O.; Noureddine, B. Chemical composition, antibacterial and antioxidant activities of some essential oils against multidrug resistant bacteria. Eur. J. Integr. Med. 2020, 35, 1–24. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- Yoshida, N.C.; Saffran, F.P.; Lima, W.G.; Freire, T.V.; Siqueira, J.M.; Garcez, W.S. Chemical characterization and bioherbicidal potential of the essential oil from the leaves of Unonopsis guatterioides (A.DC.) R.E.Fr. (Annonaceae). Nat. Prod. Res. 2019, 33, 1–5. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleiria, S. Antimicrobial activity of some essential oils—Present status and Future perpectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Solarte, A.L.; Astorga, R.J.; Aguiar, F.; Relaño-Galán, A.; Maldonado, A.; Huerta, B. Combination of antimicrobials and essential oils as an alternative for the control of Salmonella enterica multiresistant strains related to foodborne disease. Foodborne Pathog. Dis. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.-J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strassburg, B.B.; Latawiec, A.; Balmford, A. Brazil: Urgent action on Cerrado extinctions. Nature 2016, 540, 199. [Google Scholar] [CrossRef] [PubMed]
- Forzza, R.C.; Baumgratz, J.F.A.; Bicudo, C.E.M.; Canhos, D.A.L.; Carvalho, A.A.; Costa, A.F.; Costa, D.P.; Hopkins, M.; Leitman, P.M.; Lohmann, L.G.; et al. Catálogo de Plantas e Fungos do Brasil, 1st ed.; Andrea Jakobsson Estúdio Editorial: Rio de Janeiro, Brazil, 2010; ISBN 978-85-88742-43-7. [Google Scholar]
- Sano, S.M.; Almeida, S.P.; Ribeiro, J.F. Cerrado: Ecologia e Flora, 1st ed.; Embrapa Cerrados: Brasília, Brazil, 2008; ISBN 9788573834215. [Google Scholar]
- Strassburg, B.B.N.; Brooks, T.; Feltran-Barbieri, R.; Iribarrem, A.; Crouzeilles, R.; Loyola, R.; Latawiec, A.E.; Filho, F.J.B.O.; Scaramuzza, C.A.M.; Scarano, F.R.; et al. Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 2017, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Garcez, F.R.; Garcez, W.S.; Yoshida, N.C.; Figueiredo, P.O. A Diversidade dos constituintes químicos da flora de Mato Grosso do Sul e sua relevância como fonte de substâncias bioativas. Rev. Virtual Quim. 2016, 8, 97–126. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 811. ISBN 978-193-263-321-4. [Google Scholar]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Desvleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta. Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, S.; Dohoo, I.R.; Barkema, H.W.; Descôteaux, L.; Devries, T.J.; Reyher, K.K.; Roy, J.P.; School, D.T. Manageable risk factors associated with the lactational incidence, elimination, and prevalence of Staphylococcus aureus intramammary infections in dairy cows. J. Dairy Sci. 2012, 95, 1283–1300. [Google Scholar] [CrossRef] [Green Version]
- Kümmel, J.; Stessl, B.; Gonano, M.; Walcher, G.; Bereuter, O.; Fricker, M.; Grunert, T.; Wagner, M.; Ehling-Schulz, M. Staphylococcus aureus entrance into the dairy chain: Tracking S. aureus from dairy cow to cheese. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Almeida, J.R.G.S.; Facanali, R.; Vieira, M.A.R.; Marques, M.O.M.; Lúcio, A.S.S.C.; Lima, E.O.; Barbosa-Filho, J.M. Composition and Antimicrobial Activity of the Leaf Essential Oils of Duguetia gardneriana Mart. And Duguetia moricandiana Mart. (Annonaceae). J. Essent. Oil Res. 2010, 22, 275–278. [Google Scholar] [CrossRef]
- Yoo, H.J.; Jwa, S.K. Inhibitory effects of β-caryophyllene on Streptococcus mutans biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Neta, M.C.S.; Vittorazzi, C.; Guimarães, A.C.; Martins, J.D.L.; Fronza, M.; Endringer, D.C.; Scherer, R. Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24h time–kill curve studies. Pharm. Biol. 2017, 55, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.J.; Yang, J.Y.; Lee, M.H.; Kim, H.W.; Kwon, H.J.; Park, M.; Kim, S.K.; Park, S.Y.; Kim, J.B. Inhibitory effects of β-Caryophyllene on Helicobacter pylori infection in vitro and in vivo. Int. J. Mol. Sci. 2020, 21, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieri, F.A.; Souza, M.C.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet. Res. 2016, 12, 216. [Google Scholar] [CrossRef] [Green Version]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta. 2015, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.G.; Dognini, J.; Begnini, I.M.; Rebelo, R.A.; Verdi, M.; Gasper, A.L.D.; Dalmarco, E.M. Chemical characterization of essential oils from Drimys angustifolia Miers (Winteraceae) and antibacterial activity of their major compounds. J. Braz. Chem. Soc. 2013, 24, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Shih, M.-K.; Lai, Y.-H.; Lin, C.-M.; Chen, Y.-W.; Hou, Z.-T.; Hou, C.-Y. A novel application of terpene compound α-pinene for alternative use of sulfur dioxide-free white wine. Int. J. Food Prop. 2020, 23, 520–532. [Google Scholar] [CrossRef] [Green Version]
- Murakami, C.; Lago, J.H.G.; Perazzo, F.F.; Ferreira, K.S.; Lima, M.E.L.; Moreno, P.R.H.; Young, M.C.M. Chemical Composition and Antimicrobial Activity of Essential Oils from Chromolaena laevigataduring Flowering and Fruiting Stages. Chem. Biodivers. 2013, 10, 621–627. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Cardoso, C.A.L.; Ré-Poppi, N.; Melo, A.M.; Vieira, M.C.; Honda, N.K.; Coelho, R.G. Gas Chromatography-Mass Spectrometry (GC-MS) and evaluation of antioxidant and antimicrobial activities of essential oil of Campomanesia adamantium (Cambess.) O. Berg (Guavira). Braz. J. Pharm. Sci. 2009, 45, 767–776. [Google Scholar] [CrossRef]
- Santos, A.L.; Polidoro, A.S.; Cardoso, C.A.L.; Batistote, M.; Vieira, M.C.; Jacques, R.A.; Camarão, E.B. GC×GC/qMS analyses of Campomanesia guazumifolia (Cambess.) O. Berg essential oils and their antioxidant and antimicrobial activity. Nat. Prod. Res. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Durigan, G.; Pilon, N.A.L.; Assis, G.B.; Souza, F.M.; Baitello, J.B. Plantas Pequenas do Cerrado: Biodiversidade Negligenciada, 1st ed.; Secretaria do Meio Ambiente do Estado de São Paulo: São Paulo, Brazil, 2018; p. 720. ISBN 978-85-8156-030-4. [Google Scholar]
- Messias, M.C.T.B.; Menegatto, M.F.; Prado, A.C.C.; Santos, B.R.; Guimarães, M.F.M. Uso popular de plantas medicinais e perfil socioeconômico dos usuários: Um estudo em área urbana em Ouro Preto, MG, Brasil. Rev. Bras. Plantas. Med. 2015, 17, 76–104. [Google Scholar] [CrossRef]
- Kataoka, V.M.F.; Cardoso, C.A.L. Avaliação do perfil cromatográfico obtidos por CLAE-DAD e da atividade antioxidante das folhas de espécies Campomanesia sessiliflora (O. Berg) Mattos e Campomanesia xanthocarpa O. Berg. Ver. Bras. Plantas Med. 2013, 15, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R.L.G.; Souza, V.C.; Ferrucci, M.S.; Flores, T.B. Revisão taxonômica de Matayba sect. Matayba (Sapindaceae, Cupanieae). Rodriguésia 2017, 68, 411–443. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.K.D.; Silva, M.A.P.; Menezes, I.R.A.; Ribeiro, D.A.; Bezerra, L.R.; Souza, M.M.A. Ethnopharmacology of medicinal plants of carrasco, northeastern Brazil. J. Ethnopharmacol. 2014, 157, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.F.; Kinoshita, L.S. Myrsine (Myrsinoideae-Primulaceae) no sudeste e sul do Brasil. Rodriguésia 2015, 66, 167–189. [Google Scholar] [CrossRef]
- Calle, J.; Olarte, J.; Pinzon, R.; Ospina, L.F.; Mendoza, M.C.; Orozco, M.J. Alterations in the reproduction of mice induced by rapanone. J. Ethnopharmacol. 2000, 71, 521–525. [Google Scholar] [CrossRef]
- Ospina, L.F.; Calle, J.; Arteaga, L.; Pinzón, R.; Alcaraz, M.J.; Payá, M. Inhibition of acute and chronic inflammatory responses by the hydroxybenzoquinonic derivative rapanone. Planta. Med. 2001, 67, 791–795. [Google Scholar] [CrossRef]
- Manda, B.R.; Prasad, A.N.; Thatikonda, N.R.; Lacerda, J.R.V.; Barbosa, L.R.; Santos, H.; Romão, W.; Pavan, F.R.; Ribeiro, C.M.; Santos, E.A.; et al. Synthesis, antibacterial and antitubercular evaluation of cardanol and glycerol-based β-amino alcohol derivatives. J. Braz. Chem. Soc. 2018, 29, 639. [Google Scholar] [CrossRef]
Sample Availability: Samples of the essential oils are available from the authors. |
| MIC (µg·mL−1) | |||||||
|---|---|---|---|---|---|---|---|
| Plant species/Essential Oil | E. coli NEWP0022 | E. coli NEWP 0018 | S. aureus NEWP 0023 | P. aeruginosa NEWP0027 | Staphylococcus sp. 841 | Staphylococcus sp. 873 | Salmonella Typhi 905 |
| Chromolaena squalida | >1000 | >1000 | 125 | 500 | 7.80 | 250 | >1000 |
| Campomanesia sessiliflora | 500 | >1000 | 250 | 500 | 31.25 | 250 | 500 |
| Myrsine guianensis | 500 | >1000 | 500 | 500 | 31.25 | 500 | >1000 |
| Matayba guianensis | 500 | 500 | 500 | 500 | 125 | 500 | >1000 |
| Siparuna guianensis | >1000 | >1000 | 500 | 500 | 500 | 500 | >1000 |
| Ocotea minarum | 500 | >1000 | 250 | 500 | 250 | 250 | >1000 |
| Endlicheria paniculata | 500 | >1000 | 500 | 500 | 500 | 250 | >1000 |
| Gentamincin | ≤0.5 | ≤0.5 | ≤0.5 | 3.5 | ≤0.5 | 3.5 | ≤0.5 |
| Compounds | Molecular Formula | RI + Exp | RI ++ Ref. | Chromolaena squalida | Campomanesia sessiliflora | Myrsine guianensis | Matayba guianensis |
|---|---|---|---|---|---|---|---|
| Peak Area (%) | |||||||
| α-Thujene 1 | C10H16 | 922 | 924 | 0.27 | 0.27 | 0.01 | - |
| α-Pinene 2 | C10H16 | 930 | 932 | 1.00 | 38.65 | - | - |
| Camphene 3 | C10H16 | 944 | 946 | - | 0.03 | - | - |
| Sabinene 4 | C10H16 | 968 | 969 | 0.06 | - | - | - |
| β-Pinene 5 | C10H16 | 972 | 974 | 1.38 | 0.69 | - | - |
| Myrcene 6 | C10H16 | 986 | 988 | 1.35 | - | - | - |
| α-Phellandrene 7 | C10H16 | 1002 | 1002 | 0.04 | 0.20 | - | - |
| α-Terpinene 8 | C10H16 | 1013 | 1014 | 0.10 | - | - | - |
| o-Cymene 9 | C10H14 | 1021 | 1022 | 0.14 | 0.10 | - | - |
| Limonene 10 | C10H16 | 1024 | 1024 | 2.18 | 2.10 | - | - |
| (Z)-β-Ocimene 11 | C10H16 | 1033 | 1032 | 0.14 | - | - | - |
| (E)-β-Ocimene 12 | C10H16 | 1043 | 1044 | 4.77 | - | - | - |
| γ-Terpinene 13 | C10H16 | 1054 | 1054 | 0.30 | 0.17 | - | - |
| Terpinolene 14 | C10H16 | 1085 | 1086 | 0.76 | 0.07 | - | - |
| Eucalyptol 15 | C10H18O | 1027 | 1026 | - | 2.74 | 0.02 | - |
| Linalool 16 | C10H18O | 1097 | 1095 | 0.15 | 1.67 | 0.05 | - |
| Terpinen-4-ol 17 | C10H18O | 1173 | 1174 | 0.36 | 0.04 | - | - |
| α-Terpineol 18 | C10H18O | 1188 | 1186 | 0.19 | 0.37 | - | - |
| δ-Elemene 19 | C15H24 | 1332 | 1335 | 0.91 | - | - | 2.73 |
| α-Cubebene 20 | C15H24 | 1344 | 1348 | 0.36 | - | 0.44 | 0.14 |
| Cyclosativene 21 | C15H24 | 1362 | 1369 | 0.22 | - | - | - |
| α-Ylangene 22 | C15H24 | 1366 | 1373 | 0.37 | - | 0.59 | 0.24 |
| Isoledene 23 | C15H24 | 1368 | 1374 | 0.19 | 0.32 | - | 0.47 |
| α-Copaene 24 | C15H24 | 1371 | 1374 | 1.95 | 0.11 | 7.00 | 1.34 |
| β-Bourbonene 25 | C15H24 | 1380 | 1387 | 0.32 | - | 0.30 | 0.21 |
| β-Elemene 26 | C15H24 | 1387 | 1389 | 1.26 | 0.19 | 0.55 | 1.95 |
| α-Gurjunene 27 | C15H24 | 1405 | 1409 | 0.31 | 1.08 | 0.06 | 0.21 |
| Isocaryophyllene 28 | C15H24 | 1414 | 1408 | - | - | 21.81 | - |
| β-Caryophyllene 29 | C15H24 | 1415 | 1417 | 12.54 | 13.35 | - | 15.45 |
| Aromadendrene 30 | C15H24 | 1434 | 1439 | 0.32 | 12.52 | 7.82 | 1.28 |
| α-Humulene 31 | C15H24 | 1448 | 1452 | 1.92 | 1.69 | 7.80 | 2.10 |
| (E)-β-Farnesene 32 | C15H24 | 1452 | 1454 | - | - | 0.68 | - |
| α-Patchoulene 33 | C15H24 | 1456 | 1454 | 1.26 | 3.57 | 0.77 | 0.81 |
| 10-β-Cadina-1(6),4-diene 34 | C15H24 | 1468 | 1475 | 1.38 | - | - | - |
| α-Amorphene 35 | C15H24 | 1473 | 1483 | - | - | - | 1.37 |
| Germacrene D 36 | C15H24 | 1478 | 1480 | 12.74 | - | 1.95 | 28.39 |
| β-Selinene 37 | C15H24 | 1482 | 1489 | 0.65 | 0.47 | 3.51 | 0.33 |
| γ-Muurolene 38 | C15H24 | 1482 | 1478 | 4.85 | 0.42 | 7.05 | - |
| Valencene 39 | C15H24 | 1490 | 1496 | - | - | 11.52 | - |
| Viridiflorene 40 | C15H24 | 1490 | 1496 | 14.32 | 7.55 | - | - |
| Bicyclogermacrene 41 | C15H24 | 1493 | 1500 | - | - | 0.37 | 31.32 |
| α-Muurolene 42 | C15H24 | 1496 | 1500 | 3.29 | - | 0.64 | 0.56 |
| γ-Cadinene 43 | C15H24 | 1510 | 1513 | 4.06 | 0.77 | 4.45 | - |
| δ-Cadinene 44 | C15H24 | 1519 | 1522 | 11.60 | - | 11.03 | 0.76 |
| trans-Cadina-1,4-diene 45 | C15H24 | 1527 | 1533 | 0.73 | - | - | - |
| α-Calacorene 46 | C15H24 | 1539 | 1544 | - | 0.10 | - | - |
| Germacrene B 47 | C15H24 | 1552 | 1559 | 2.49 | - | 3.02 | 5.19 |
| Elemol 48 | C15H26O | 1545 | 1548 | - | 0.14 | - | - |
| Spathulenol 49 | C15H24O | 1574 | 1577 | - | 6.46 | 2.21 | 2.29 |
| (E)-Nerolidol 50 | C15H26O | 1559 | 1561 | - | - | 4.71 | - |
| Viridiflorol 51 | C15H26O | 1598 | 1592 | 8.74 | 1.44 | 0.89 | 1.83 |
| 5-epi-7-epi-α-Eudesmol 52 | C15H26O | 1598 | 1607 | - | - | - | 0.67 |
| γ-Eudesmol 53 | C15H26O | 1628 | 1630 | - | 0.59 | - | - |
| epi-α-Muurolol 54 | C15H26O | 1637 | 1640 | - | 0.62 | - | - |
| δ-cadinol 55 | C15H26O | 1643 | 1644 | - | 0.12 | 0.48 | - |
| β-Eudesmol 56 | C15H26O | 1649 | 1649 | - | 1.36 | 0.19 | - |
| Identified compounds (%) | 99.97 | 99.97 | 99.92 | 99.64 | |||
| Monoterpene Hydrocarbons (%) | 12.49 | 42.28 | 0.01 | - | |||
| Oxygenated Monoterpenes (%) | 0.70 | 4.82 | 0.07 | - | |||
| Sesquiterpene Hydrocarbons (%) | 78.04 | 42.14 | 91.36 | 94.85 | |||
| Oxygenated Sesquiterpenes (%) | 8.74 | 10.73 | 8.48 | 4.79 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus, G.S.; Micheletti, A.C.; Padilha, R.G.; de Souza de Paula, J.; Alves, F.M.; Leal, C.R.B.; Garcez, F.R.; Garcez, W.S.; Yoshida, N.C. Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug−Resistant Foodborne Microorganisms. Molecules 2020, 25, 3296. https://doi.org/10.3390/molecules25143296
de Jesus GS, Micheletti AC, Padilha RG, de Souza de Paula J, Alves FM, Leal CRB, Garcez FR, Garcez WS, Yoshida NC. Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug−Resistant Foodborne Microorganisms. Molecules. 2020; 25(14):3296. https://doi.org/10.3390/molecules25143296
Chicago/Turabian Stylede Jesus, Genilson Silva, Ana Camila Micheletti, Rafael Gonçalves Padilha, Jessica de Souza de Paula, Flavio Macedo Alves, Cassia Rejane Brito Leal, Fernanda Rodrigues Garcez, Walmir Silva Garcez, and Nidia Cristiane Yoshida. 2020. "Antimicrobial Potential of Essential Oils from Cerrado Plants against Multidrug−Resistant Foodborne Microorganisms" Molecules 25, no. 14: 3296. https://doi.org/10.3390/molecules25143296