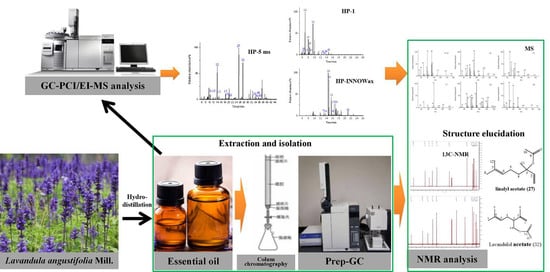
Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
2.2. Improvements in the Fraction Collector of pGC
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Extraction of the Essential Oil
3.3. Gas Chromatography Analysis
3.4. Isolation and Structure Elucidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabata, J.; Teshiba, M.; Shimizu, N.; Sugie, H. Mealybug mating disruption by a sex pheromone derived from lavender essential oil. J. Essent. Oil Res. 2015, 27, 232–237. [Google Scholar] [CrossRef]
- Maria, L.-B. Lavender The Genus Lavandula; Taylor & Francis: London, UK; New York, NY, USA, 2002; pp. 2–86. [Google Scholar]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Imane, M.M.; Houda, F.; Amal, A.H.S.; Kaotar, N.; Mohammed, T.; Imane, R.; Farid, H. Phytochemical composition and antibacterial activity of moroccan Lavandula angustifolia mill. J. Essent. Oil. Bear. Plants 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Bialon, M.; Krzysko-Lupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef] [Green Version]
- Ali-Shtayeh, M.S.; Abu-Zaitoun, S.Y.; Dudai, N.; Jamous, R.M. Downy lavender oil: A promising source of antimicrobial, antiobesity, and anti-alzheimer’s disease agents. Evid.-Based Complementary Altern. 2020, 2020, 5679408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sarar, A.S.; Hussein, H.I.; Abobakr, Y.; Bayoumi, A.E.; Al-Otaibi, M.T. Fumigant toxicity and antiacetylcholinesterase activity of Saudi Mentha longifolia and Lavandula dentata species against Callosobruchus maculatus (F.)(Coleoptera: Bruchidae). Turk. Entomoloji Derg-Turk. 2014, 38, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Romeo, F.V.; De Luca, S.; Piscopo, A.; Poiana, M. Antimicrobial effect of some essential oils. J. Essent. Oil Res. 2008, 20, 373–379. [Google Scholar] [CrossRef]
- Tomi, K.; Kitao, M.; Murakami, H.; Matsumura, Y.; Hayashi, T. Classification of lavender essential oils: Sedative effects of Lavandula oils. J. Essent. Oil Res. 2018, 30, 56–68. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of Lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandula angustifolia and lavandula latifolia essential oils from spain: Aromatic profile and bioactivities. Planta Med. 2015, 82, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Woronuk, G.; Demissie, Z.; Rheault, M.; Mahmoud, S. Biosynthesis and therapeutic properties of lavandula essential oil constituents. Planta Med. 2011, 77, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Adenubi, O.T.; Ahmed, A.S.; Fasina, F.O.; Mcgaw, L.J.; Eloff, J.N.; Naidoo, V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind. Crop. Prod. 2018, 123, 779–806. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jozwiak, K.; Waksmundzka-Hajnos, M.; Ciesla, L. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Francisco, C.G.; Freire, R.; Hernández, R.; Salazar, J.A.; Suárez, E.; Cortés, M. Carbon-13 NMR study of (20,24)-epoxydammarane triterpenes. Magn. Reson. Chem. 1984, 22, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.W.; Choi, S.H.; Lim, S. Combined Effects of the Essential Oil from Pelargonium graveolens with Antibiotics against Streptococcus pneumonia. Nat. Prod. Sci. 2007, 13, 342–346. [Google Scholar]
- Schulz, S.; Steffensky, M.; Roisin, Y. Identification and synthesis of elymniafuran, a new monoterpene from the butterfly elymnias thryallis. Liebigs Ann. 1996, 6, 941–946. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Lee, K.R. Phytochemical constituents of amomum xanthioides. Nat. Prod. Sci. 2009, 15, 44–49. [Google Scholar]
- Miyazawa, M.; Nankai, H.; Kameoka, H. Enantioselective cyclization of (+/-)-lavandulol to (-)-(2S,4S)-1,5-epoxy-5-methyl-2-(1-methylethenyl)-4-hexanol by Glomerella cingulate. Nat. Prod. Lett. 1997, 9, 249–252. [Google Scholar] [CrossRef]
- Queiroga, C.L.; Ferracini, V.L.; Marsaioli, A.J. Three new oxygenated cadinanes from baccharis species. Phytochemistry 1996, 42, 1097–1103. [Google Scholar] [CrossRef]
- Guan, J.; Zhao, W.J.; Wei, J.J.; Gao, L. Studies on the chemical constituents of the extracts of lavender flowers from supercritical CO2 fluid. Lishizhen Med. Mater. Med. 2009, 20, 890–891. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zeisberg, R.; Klein, E. 13C-NMR-spektren von monoterpenen. Magn. Reson. Chem. 2005, 7, 426–432. [Google Scholar] [CrossRef]
- Srivastava, P.L.; Daramwar, P.P.; Krithika, R.; Pandreka, A.; Shankar, S.S.; Thulasiram, H.V. Functional characterization of novel sesquiterpene synthases from indian sandalwood, santalum album. Sci. Rep. 2015, 5, 10095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umlauf, D.; Zapp, J.; Becker, H.; Adam, K.P. Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae). Phytochemistry 2004, 65, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, I.; Kreuzenbeck, N.; Beemelmanns, C.; Dickschat, J. Mechanistic characterization of three sesquiterpene synthases from the termiteassociated fungus Termitomyces. Org. Biomol. Chem. 2019, 17, 3348–3355. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Ganzon, J.; Hofilena, J.; Tamboong, B.; Rideout, J.A. A new furanoid diterpene from Caesalpinia pulcherrima. Chem. Pharm. Bull. 2003, 51, 1208–1210. [Google Scholar] [CrossRef] [Green Version]
- Weinert, B.; Wüst, M.; Mosandl, A.; Hanssum, H. Stereoisomeric flavour compounds. LXXVIII. Separation and structure elucidation of the pyranoid linalool oxide stereoisomers using common gas chromatographic phases, modified cyclodextrin phases and nuclear magnetic resonance spectroscopy. Phytochem. Anal. 1998, 9, 10–13. [Google Scholar] [CrossRef]
- Strauss, C.R.; Wilson, B.; Rapp, A.; Guentert, M.; Williams, P.J. New monoterpene ethyl ethers in grape wines and brandies. J. Agric. Food Chem. 1985, 33, 706–708. [Google Scholar] [CrossRef]
- Mookherjee, B.D.; Trenkle, R.W. Isolation, identification, and biogenesis of bifunctional compounds in lavandin oil. J. Agric. Food Chem. 1973, 21, 298–302. [Google Scholar] [CrossRef]
- Ngo, K.S.; Brown, G.D. Santalane and isocampherenane sesquiterpenoids from illicium tsangii. Phytochemistry 1999, 50, 1213–1218. [Google Scholar] [CrossRef]
- Singh, D.; Kumar Chaudhuri, P.; Darokar, M.P. New antiproliferative tricyclic sesquiterpenoid from the leaves of Ocimum sanctum. Helv. Chim. Acta 2014, 97, 708–711. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, W.Q.; Wang, F.; Liu, J.K.; Gao, J.M. Chemical component from allelopathic cultivar sunflower leaves. Acta Bot. Boreali-Occident. Sin. 2007, 27, 532–536. [Google Scholar] [CrossRef]
- De Andrade, D.J.; Pattaro, F.C.; De Oliveira, C.A.L. Lime sulfur residue on acaricide efficiency in the control of Brevipalpus phoenicis. Cienc. Rural 2011, 41, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Adaszynska-Skwirzynska, M.; Dzieciol, M. Comparison of chemical composition and antimicrobial activity of essential oils obtained from different cultivars and morphological parts of Lavandula angustifolia. J. Essent. Oil Bear. Plants 2018, 21, 1532–1541. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
Sample Availability: The data that support the findings of this study are available from the corresponding author upon reasonable request. |
| NO. | Compound Name | Molecular Formula | Lit. RIHP-5 a | Exp. RIHP-5 ms b | Exp. RIHP-1 | Exp. RIHP-INNOWax | Relative Content/% c | Identified Methods | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Camphene | C10H16 | 952 | 943 | - | 1088 | 0.41 | GC-MS, RI | |
| 2 | β-Myrcene | C10H16 | 991 | 989 | 982 | 1168 | 0.60 | GC-MS, RI | |
| 3 | p-Cymene | C10H14 | 1025 | 1035 | 1014 | 1285 | 0.23 | GC-MS, RI | |
| 4 | β-Cymene | C10H14 | 1023 | 1021 | 1011 | 1290 | 0.61 | GC-MS, RI | |
| 5 | Limonene | C10H16 | 1030 | 1024 | - | 1217 | 0.58 | GC-MS, RI | |
| 6 | Cineole | C10H18O | 1032 | 1026 | 1021 | 1219 | 1.05 | GC-MS, RI | |
| 7 | β-cis-Ocimene | C10H16 | 1038 | 1033 | 1026 | 1240 | 3.31 | GC-MS, RI | |
| 8 | β-trans-Ocimene | C10H16 | 1049 | 1044 | 1038 | 1259 | 1.41 | GC-MS, RI | |
| 9 | cis-Linalool oxide | C10H18O2 | 1074 | 1069 | 1059 | 1455 | 0.49 | GC-MS, RI, 13C-NMR | |
| 10 | trans-Linalool oxide | C10H18O2 | 1086 | 1069 | 1074 | 1484 | GC-MS, RI, 13C-NMR | ||
| 11 | α-Terpinolen | C10H16 | 1088 | 1086 | - | 1301 | 0.63 | GC-MS, RI | |
| 12 | Linalool | C10H18O | 1099 | 1102 | 1090 | 1545 | 19.71 | GC-MS, RI, 13C-NMR | |
| 13 | Hotrienol | C10H16O | 1107 | 1105 | - | - | 0.46 | GC-MS, RI, 13C-NMR | |
| 14 | 1-Pentylallyl acetate | C10H18O2 | 1111 | 1114 | 1095 | 1380 | 1.01 | GC-MS, RI | |
| 15 | (4E,6Z)-allo-Ocimene | C10H16 | 1131 | 1128 | - | - | 0.25 | GC-MS, RI | |
| 16 | Camphor | C10H16O | 1145 | 1140 | 1123 | - | 0.42 | GC-MS, RI | |
| 17 | (−)-Borneol | C10H18O | 1167 | 1161 | 1152 | 1723 | 1.78 | GC-MS, RI, 13C-NMR | |
| 18 | Lavandulol | C10H18O | 1170 | 1166 | - | 1681 | 0.48 | GC-MS, RI, 13C-NMR | |
| 19 | 2,2,6-trimethyl-6-vinyltetrahydro-2H-pyran-3-ol | C10H18O2 | - | 1166 | - | - | - | GC-MS, RI, 13C-NMR | |
| 20 | 4-Terpineol | C10H18O | 1177 | 1173 | 1165 | 1621 | 0.41 | GC-MS, RI | |
| 21 | Cryptone | C9H14O | 1184 | 1182 | 1160 | 1715 | 0.62 | GC-MS, RI, 13C-NMR | |
| 22 | α-Terpineol | C10H18O | 1189 | 1187 | 1176 | 1712 | 3.61 | GC-MS, RI | |
| 23 | Verbenone | C10H14O | 1205 | 1204 | 1185 | - | 0.22 | GC-MS, RI | |
| 24 | Bornyl formate | C11H18O2 | 1226 | 1225 | - | - | 0.41 | GC-MS, RI | |
| 25 | Neryl alcohol | C10H18O | 1228 | 1229 | 1213 | 1804 | 0.49 | GC-MS, RI | |
| 26 | p-Cumic aldehyde | C10H12O | 1239 | 1238 | - | 1823 | 0.72 | GC-MS, RI | |
| 27 | Linalyl acetate | C12H20O2 | 1257 | 1263 | 1242 | 1564 | 26.61 | GC-MS, RI, 13C-NMR | |
| 28 | Phellandral | C10H16O | 1276 | 1273 | 1254 | - | 0.35 | GC-MS, RI | |
| 29 | 3,7-dimethylocta-1,7-diene-3,6-diol | C10H18O | - | 1276 | - | - | - | GC-MS, RI, 13C-NMR | |
| 30 | Bornyl acetate | C12H20O2 | 1285 | 1285 | - | - | 0.71 | GC-MS, RI | |
| 31 | Cuminol | C10H14O | 1289 | 1290 | - | - | 0.33 | GC-MS, RI | |
| 32 | Lavandulol acetate | C12H20O2 | - | 1295 | 1273 | 1612 | 12.68 | GC-MS, RI, 13C-NMR | |
| 33 | (E)-7-hydroxy-3,7-dimethylocta-1,5-dien-ylacetate | C12H20O3 | - | 1344 | - | - | - | GC-MS, RI, 13C-NMR | |
| 34 | Nerol acetate | C12H20O2 | 1364 | 1369 | 1343 | 1735 | 1.07 | GC-MS, RI | |
| 35 | Geranyl acetate | C12H20O2 | 1387 | 1387 | 1361 | 1765 | 1.75 | GC-MS, RI | |
| 36 | β-Caryophyllen | C15H24 | 1419 | 1416 | 1418 | 1635 | 1.75 | GC-MS, RI | |
| 37 | α-Santalene | C15H24 | 1420 | 1419 | - | - | 0.83 | GC-MS, RI | |
| 38 | (E)-β-Fanesene | C15H24 | 1457 | 1459 | 1447 | 1672 | 0.49 | GC-MS, RI, 13C-NMR | |
| 39 | d-Germacrene | C15H24 | 1481 | 1480 | 1476 | - | 0.31 | GC-MS, RI, 13C-NMR | |
| 40 | γ-Cadinene | C15H24 | 1513 | 1514 | 1509 | - | 0.23 | GC-MS, RI, 13C-NMR | |
| 41 | 11-hydroxy-α-santal-9-ene | C15H24O | - | 1519 | - | - | - | GC-MS, RI, 13C-NMR | |
| 42 | 2-Methyl-1-(4-methylphenyl)-3-buten-1-ol | C12H16O | - | 1519 | - | - | 0.52 | GC-MS,RI | |
| 43 | β-Caryophyllene oxide | C15H24O | 1581 | 1585 | 1571 | 2042 | 3.65 | GC-MS, RI, 13C-NMR | |
| 44 | Cedrelanol | C15H26O | 1640 | 1643 | 1628 | 2206 | 0.89 | GC-MS,RI | |
| 45 | (3S,6R,9R)-2-(hydroxymethyl)-5,5,9-trimethyltricyclo [7.2.0.0(3,6)]undecan-2-ol | C15H26O2 | - | 1811 | - | - | - | GC-MS, RI, 13C-NMR | |
| 46 | (6R,10R)-6,10,14-Trimethyl-2-pentadecanone | C18H36O | - | 1846 | - | - | - | GC-MS, RI, 13C-NMR | |
| 47 | S8 | - | 2031 | - | - | - | GC-MS | ||
| Component group | |||||||||
| Monoterpene hydrocarbons | 8.03 | ||||||||
| Sesquiterpene hydrocarbons | 3.61 | ||||||||
| Oxygenated monoterpenes | 31.53 | ||||||||
| Oxygenated sesquiterpenes | 4.54 | ||||||||
| Esters | 43.23 | ||||||||
| Others | 1.14 | ||||||||
| Total identified | 92.08 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, G.; Bai, X.; Aimila, A.; Aisa, H.A.; Maiwulanjiang, M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules 2020, 25, 3166. https://doi.org/10.3390/molecules25143166
Dong G, Bai X, Aimila A, Aisa HA, Maiwulanjiang M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules. 2020; 25(14):3166. https://doi.org/10.3390/molecules25143166
Chicago/Turabian StyleDong, Guangyao, Xiaohui Bai, Aoken Aimila, Haji Akber Aisa, and Maitinuer Maiwulanjiang. 2020. "Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC" Molecules 25, no. 14: 3166. https://doi.org/10.3390/molecules25143166