Optimising the Hydraulic Retention Time in a Pilot-Scale Microbial Electrolysis Cell to Achieve High Volumetric Treatment Rates Using Concentrated Domestic Wastewater
Abstract
:1. Introduction
2. Results
2.1. Start-Up and Operation
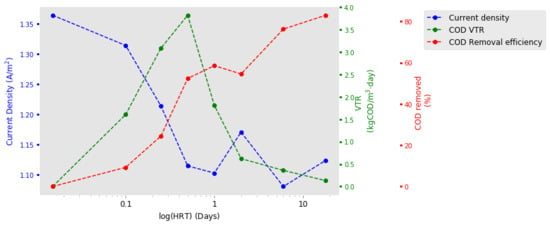
2.2. HRT Optimisation
2.3. Energetic Cost
2.4. The Effect of Influent COD Concentrations
2.5. Tank Design
2.6. Variability
3. Discussion
4. Materials and Methods
4.1. Reactor Configuration
4.2. Experimental Site and Operational Conditions
4.3. Analytical Methods
4.4. Inoculation and Batch Mode
4.5. Experimental Conditions
4.6. Calculations
4.7. Tank Design
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.E.; Dolfing, J.; Jones, C.; Curtis, T.P.; Heidrich, E.S. Low Temperature Domestic Wastewater Treatment in a Microbial Electrolysis Cell with 1 m 2 Anodes: Towards System Scale-Up. Fuel Cells 2017, 17, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Ieropoulos, I.A. Recent advancements in real-world microbial fuel cell applications. Curr. Opin. Electrochem. 2018, 11, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z. Demystifying terms for understanding bioelectrochemical systems towards sustainable wastewater treatment. Curr. Opin. Electrochem. 2020, 19, 14–19. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Leicester, D.D.; Heidrich, E.S.; Alonso, R.M.; Mateos, R.; Escapa, A. Bioelectrochemical Systems for Energy Valorization of Waste Streams. In Energy Systems and Environment; InTech: London, UK, 2018. [Google Scholar]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. from laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Rossi, R.; Jones, D.; Myung, J.; Zikmund, E.; Yang, W.; Gallego, Y.A.; Pant, D.; Evans, P.J.; Page, M.A.; Cropek, D.M.; et al. Evaluating a multi-panel air cathode through electrochemical and biotic tests. Water Res. 2019, 148, 51–59. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, W.; Guo, J.; Sun, D.; Pan, B.; Ye, Y.; Ding, W.; Huang, H.; Li, F. Effects of hydraulic pressure on the performance of single chamber air-cathode microbial fuel cells. Biosens. Bioelectron. 2014, 56, 264–270. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Dolfing, J.; Scott, K.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar] [CrossRef]
- Isabel San-Martín, M.; Mateos, R.; Carracedo, B.; Escapa, A.; Morán, A. Pilot-scale bioelectrochemical system for simultaneous nitrogen and carbon removal in urban wastewater treatment plants. J. Biosci. Bioeng. 2018, 126, 758–763. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Sotres, A.; Alonso, R.M.; Díaz-Marcos, J.; Morán, A.; Escapa, A. Assessing anodic microbial populations and membrane ageing in a pilot microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 17304–17315. [Google Scholar] [CrossRef]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Brown, R.K.; Harnisch, F.; Wirth, S.; Wahlandt, H.; Dockhorn, T.; Dichtl, N.; Schröder, U. Evaluating the effects of scaling up on the performance of bioelectrochemical systems using a technical scale microbial electrolysis cell. Bioresour. Technol. 2014, 163, 206–213. [Google Scholar] [CrossRef]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gil-Carrera, L.; Escapa, A.; Mehta, P.; Santoyo, G.; Guiot, S.R.; Moran, A.; Tartakovsky, B. Microbial electrolysis cell scale-up for combined wastewater treatment and hydrogen production. Bioresour Technol 2013, 130, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Gil-Carrera, L.; Escapa, A.; Moreno, R.; Morán, A. Reduced energy consumption during low strength domestic wastewater treatment in a semi-pilot tubular microbial electrolysis cell. J. Environ. Manag. 2013, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gil-Carrera, L.; Escapa, A.; Carracedo, B.; Morán, A.; Gómez, X. Performance of a semi-pilot tubular microbial electrolysis cell (MEC) under several hydraulic retention times and applied voltages. Bioresour. Technol. 2013, 146, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Carlotta-Jones, D.I.; Purdy, K.; Kirwan, K.; Stratford, J.; Coles, S.R. Improved hydrogen gas production in microbial electrolysis cells using inexpensive recycled carbon fibre fabrics. Bioresour. Technol. 2020, 304, 122983. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal-microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Mills, N.; Pearce, P.; Farrow, J.; Thorpe, R.B.; Kirkby, N.F. Environmental & economic life cycle assessment of current & future sewage sludge to energy technologies. Waste Manag. 2014, 34, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Department of Environment Food and Rural Affairs, UK. Anaerobic Digestion Strategy and Action Plan. A Commitment to Increasing Energy from Waste through Anaerobic Digestion; Department of Energy and Climate Change: London, UK, 2011.
- Dai, Z. Developing Energy and Nutrient Mass Balances to Inform Value Recovery Options in Municipal Wastewater Treatment Systems. Ph.D. Thesis, Newcastle University, Newcastle, UK, 2019. [Google Scholar]
- Dai, Z.; Heidrich, E.S.; Dolfing, J.; Jarvis, A.P. Determination of the Relationship between the Energy Content of Municipal Wastewater and Its Chemical Oxygen Demand. Environ. Sci. Technol. Lett. 2019, 6, 396–400. [Google Scholar] [CrossRef]
- Oakley, M. Environmental engineering. Mil. Eng. 2018, 110, 55–57. [Google Scholar]
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Pant, D.; Singh, A.; Van Bogaert, G.; Gallego, Y.A.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- UKPower Compare Energy Prices Per kWh|Gas & Electric Per Unit|UKPower. Available online: https://www.ukpower.co.uk/home_energy/tariffs-per-unit-kwh (accessed on 26 March 2020).
- Business Electricity Prices Business Electricity Rates and Unit Prices per kWh. 2019. Available online: https://www.businesselectricityprices.org.uk/cost-per-kwh/ (accessed on 18 May 2020).
- Carmona-Martínez, A.A.; Trably, E.; Milferstedt, K.; Lacroix, R.; Etcheverry, L.; Bernet, N. Long-term continuous production of H2 in a microbial electrolysis cell (MEC) treating saline wastewater. Water Res. 2015, 81, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Yang, W.; Evans, P.J.; Logan, B.E. Continuous treatment of high strength wastewaters using air-cathode microbial fuel cells. Bioresour. Technol. 2016, 221, 96–101. [Google Scholar] [CrossRef]
- Aiken, D.C.; Curtis, T.P.; Heidrich, E.S. Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production. Int. J. Hydrogen Energy 2019, 44, 2426–2434. [Google Scholar] [CrossRef]
- Akman, D.; Cirik, K.; Ozdemir, S.; Ozkaya, B.; Cinar, O. Bioelectricity generation in continuously-fed microbial fuel cell: Effects of anode electrode material and hydraulic retention time. Bioresour. Technol. 2013, 149, 459–464. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Jain, A.; Aguilera, A.; He, Z. Effective control of biohythane composition through operational strategies in an innovative microbial electrolysis cell. Appl. Energy 2017, 206, 879–886. [Google Scholar] [CrossRef]
- Jiang, D.; Curtis, M.; Troop, E.; Scheible, K.; McGrath, J.; Hu, B.; Suib, S.; Raymond, D.; Li, B. A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment. Int. J. Hydrogen Energy 2011, 36, 876–884. [Google Scholar] [CrossRef]
- Yildiz, B.S. Water and wastewater treatment: Biological processes. In Metropolitan Sustainability: Understanding and Improving the Urban Environment; Woodhead Publishing: Cambridge, UK, 2012; pp. 406–428. ISBN 9780857090461. [Google Scholar]
- Recio-Garrido, D.; Perrier, M.; Tartakovsky, B. Modeling, optimization and control of bioelectrochemical systems. Chem. Eng. J. 2016, 289, 180–190. [Google Scholar] [CrossRef]
- Zhao, F.; Heidrich, E.S.; Curtis, T.P.; Dolfing, J. The effect of anode potential on current production from complex substrates in bioelectrochemical systems: A case study with glucose. Appl. Microbiol. Biotechnol. 2020, 104, 5133–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Understanding the complexity of wastewater: The combined impacts of carbohydrates and sulphate on the performance of bioelectrochemical systems. Water Res. 2020, 176, 115737. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Z.; He, D.; Li, Y.; Wu, Z. Long-term investigation of a novel electrochemical membrane bioreactor for low-strength municipal wastewater treatment. Water Res. 2015, 78, 98–110. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghangrekar, M.M.; Duteanu, N. Recent progress towards scaling up of MFCs. In Microbial Fuel Cell: A Bioelectrochemical System that Converts Waste to Watts; Springe, Cham: New Delhi, India, 2017; pp. 443–457. ISBN 9783319667935. [Google Scholar]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.F. Determining the UK’s potential for heat recovery from wastewater using steady state and dynamic modelling-preliminary results. WEENTECH Proc. Energy 2019, 5, 107–121. [Google Scholar] [CrossRef]
- Emmanuel Alepu, O.; Li, Z. Effect of Hydraulic Retention Time on Anaerobic Digestion of Xiao Jiahe Municipal Sludge. Int. J. Waste Resour. 2016, 6, 1000231. [Google Scholar] [CrossRef]
- Ichihashi, O.; Vishnivetskaya, T.A.; Borole, A.P. High-Performance Bioanode Development for Fermentable Substrates via Controlled Electroactive Biofilm Growth. ChemElectroChem 2014, 1, 1940–1947. [Google Scholar] [CrossRef]
- Council of European Communities (CEC). EU Council Directive of 21 May 1991 concerning urban waste water treatment (91/271/EEC). Off. J. Eur. Communities 1991, 135, 40–52. [Google Scholar]
- Velasquez-Orta, S.B.; Yu, E.; Katuri, K.P.; Head, I.M.; Curtis, T.P.; Scott, K. Evaluation of hydrolysis and fermentation rates in microbial fuel cells. Appl. Microbiol. Biotechnol. 2011, 90, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, U.; Choi, I.S.; Dombrowski, E.-M. Historical Development of Wastewater Collection and Treatment. In Fundamentals of Biological Wastewater Treatment; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2006; pp. 1–23. ISBN 978-3-5227-31219-1. [Google Scholar]
- Ge, Z.; He, Z. Long-term performance of a 200 L modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Linares, R.V.; Domínguez-Maldonado, J.; Rodríguez-Leal, E.; Patrón, G.; Castillo-Hernández, A.; Miranda, A.; Romero, D.D.; Moreno-Cervera, R.; Camara-chale, G.; Borroto, C.G.; et al. Scale up of microbial fuel cell stack system for residential wastewater treatment in continuous mode operation. Water 2019, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- PicoTech High-Resolution Data Acquisition|Pico Technology. Available online: https://www.picotech.com/data-logger/adc-20-adc-24/precision-data-acquisition (accessed on 31 August 2018).
- Logan, B.E. Microbial Fuel Cells; Wiley-Interscience: State College, PA, USA, 2008; ISBN 0470258586. [Google Scholar]
- Eckenfelder, W. Activated Sludge: Process Design and Control; Technomic Publishing Company, Inc.: Lancaster, PA, USA, 1998. [Google Scholar]
- Japan Sewage Works Association Design Standard for Municipal Wastewater Treatment Plants. Available online: gcus.jp/wp/wp-content/.../735735ded23d4d28db9cc4f879e8da24.pdf (accessed on 29 December 2019).
Sample Availability: Not available. |






| COD mg/L | sCOD mg/L | Phosphate mg/L | Sulphate mg/L | Ammonia mg/L | Nitrate mg/L | Nitrite mg/L | Acetate mg/L | Butyric mg/L | Formic mg/L |
|---|---|---|---|---|---|---|---|---|---|
| 4535 | 1772 | 56.8 | 180.6 | 298.3 | 8.9 | 0.8 | 274.0 | 49.2 | n/a |
| HRT (Days) | Flow Rate (L/h) | Volumetric Treatment Rate (kgCOD/m3∙day) | COD Efficiency (%) | Current Density (A/m2) | Volumetric Hydrogen Production (m3/m3∙day) | Energetic Treatment Cost (kJ/gCOD) | Coulombic Efficiency (%) | Cathodic Conversion Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| 0.015 | 100 | 0 | 0 | 1.36 ± 0.01 | 0 | n/a | 0 | 0 |
| 0.1 | 15 | 1.61 ± 2.27 | 9.3 ± 13.1 | 1.31 ± 0.07 | 0.0123 ± 0.0084 | 0.31 ± 0.44 | 2.9 ± 4.0 | 1.17 ± 0.86 |
| 0.25 | 6 | 3.09 ± 0.67 | 24.5 ± 1.6 | 1.21 ± 0.11 | 0.0155 ± 0.0076 | 0.64 ± 0.19 | 5.9 ± 1.8 | 1.16 ± 0.93 |
| 0.5 | 3 | 3.82 ± 1.94 | 52.4 ± 14.2 | 1.11 ± 0.08 | 0.0111 ± 0.0029 | 0.59 ± 0.42 | 5.6 ± 3.9 | 1.22 ± 0.25 |
| 1 | 1.5 | 1.81 ± 0.76 | 58.5 ± 7.4 | 1.10 ± 0.14 | - | 1.10 ± 0.46 | 9.9 ± 3.9 | - |
| 2 | 0.75 | 0.62 ±0.19 | 54.5 ± 2.3 | 1.17 ± 0.09 | - | 3.11 ± 0.73 | 28.0 ± 6.7 | - |
| 6 | 0.25 | 0.36 ± 0.14 | 76.2 ± 7.4 | 1.08 ± 0.04 | - | 5.25 ± 2.08 | 51.4 ± 20.5 | - |
| 18 | 0.083 | 0.13 ± 0.04 | 83.0 ± 12.9 | 1.12 ± 0.09 | - | 14.20 ± 5.04 | 131.0 ± 47.0 | - |
| Influent COD (mg/L) | Volumetric Treatment Rate (kgCOD/m3∙day) | Time of Testing | Energetic Treatment Cost (kJ/gCOD) |
|---|---|---|---|
| 4320 | 5.55 | HRT optimisation | 0.285 |
| 4153 | 5.27 | HRT optimisation | 0.36 |
| 3035 | 3.0 | HRT optimisation | 0.584 |
| 2376 | 1.43 | Tank re-circulation | 1.20 |
| 2186 | 1.47 | HRT optimisation | 1.13 |
| 950 | 0.282 | Tank re-circulation | 6.22 |
| 668 | 0.0985 | Tank re-circulation | 17.8 |
| 569 | 0.0653 | Tank re-circulation | 64.2 |
| 373 | 0.0275 | Tank re-circulation | 79.1 |
| 346 | 0.022 | Tank re-circulation | 52.1 |
| 324 | 0.0335 | Tank re-circulation | 94.1 |
| 290 | 0.0185 | Tank re-circulation | 79.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leicester, D.D.; Amezaga, J.M.; Moore, A.; Heidrich, E.S. Optimising the Hydraulic Retention Time in a Pilot-Scale Microbial Electrolysis Cell to Achieve High Volumetric Treatment Rates Using Concentrated Domestic Wastewater. Molecules 2020, 25, 2945. https://doi.org/10.3390/molecules25122945
Leicester DD, Amezaga JM, Moore A, Heidrich ES. Optimising the Hydraulic Retention Time in a Pilot-Scale Microbial Electrolysis Cell to Achieve High Volumetric Treatment Rates Using Concentrated Domestic Wastewater. Molecules. 2020; 25(12):2945. https://doi.org/10.3390/molecules25122945
Chicago/Turabian StyleLeicester, Daniel D., Jaime M. Amezaga, Andrew Moore, and Elizabeth S. Heidrich. 2020. "Optimising the Hydraulic Retention Time in a Pilot-Scale Microbial Electrolysis Cell to Achieve High Volumetric Treatment Rates Using Concentrated Domestic Wastewater" Molecules 25, no. 12: 2945. https://doi.org/10.3390/molecules25122945