Screening Antibody Libraries with Colony Assay Using scFv-Alkaline Phosphatase Fusion Proteins
Abstract
:1. Introduction
2. Results
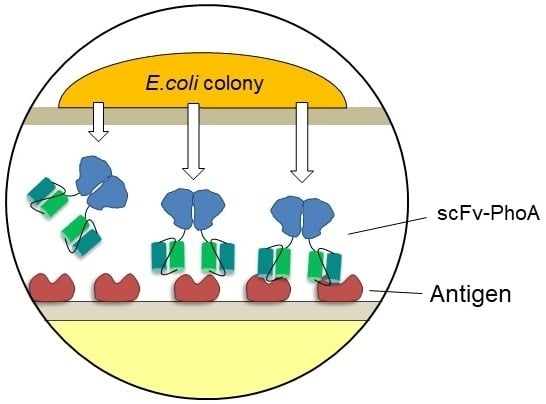
2.1. Development of the Colony Assay with scFv-PhoA
2.2. Construction of the Expression VECTOR for Screening
2.3. Screening the scFv-PhoA Library
2.4. Sequence Analysis of Selected Clones
2.5. Comparison with the scFv Library
3. Discussion
4. Materials and Methods
4.1. Immunization
4.2. Construction of scFv-PhoA and scFv Libraries
4.3. Colony Growth and scFv-PhoA and scFv Expression
4.4. Detection of Antigen Binding and Identification of Positive Clones
4.5. Expression of Positive Clones
4.6. ELISA
4.7. Sequencing
4.8. Colony PCR of Positive Clones
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Reichert, J.M. Antibodies to watch in 2015. mAbs 2015, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schirrmann, T.; Meyer, T.; Schütte, M.; Frenzel, A.; Hust, M. Phage display for the generation of antibodies for proteome research, diagnostics and therapy. Molecules (Basel, Switzerland) 2011, 16, 412–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, A.; Sblattero, D. Exploiting recombination in single bacteria to make large phage antibody libraries. Nat. Biotechnol. 2000, 18, 75–80. [Google Scholar]
- McCafferty, J.; Schofield, D. Identification of optimal protein binders through the use of large genetically encoded display libraries. Curr. Opin. Chem. Biol. 2015, 26, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Parmley, S.F.; Smith, G.P. Antibody-selectable filamentous fd phage vectors: Affinity purification of target genes. Gene 1988, 73, 305–318. [Google Scholar] [CrossRef]
- Schofield, D.J.; Pope, A.R.; Clementel, V.; Buckell, J.; Chapple, S.D.; Clarke, K.F.; Conquer, J.S.; Crofts, A.M.; Crowther, S.R.E.; Dyson, M.R.; et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol. 2007, 8, R254. [Google Scholar] [CrossRef] [Green Version]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of antibody phage display technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Matochko, W.L.; Cory Li, S.; Tang, S.K.Y.; Derda, R. Prospective identification of parasitic sequences in phage display screens. Nucleic Acids Res. 2014, 42, 1784–1798. [Google Scholar] [CrossRef] [Green Version]
- de Wildt, R.M.; Mundy, C.R.; Gorick, B.D.; Tomlinson, I.M. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat. Biotechnol. 2000, 18, 989–994. [Google Scholar] [CrossRef]
- Fitzgerald, V.; Leonard, P. Single cell screening approaches for antibody discovery. Methods 2017, 116, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Rauth, S.; Schlapschy, M.; Skerra, A. Selection of Antibody Fragments by Means of the Filter-sandwich Colony Screening Assay. In Antibody Engineering; Kontermann, R., Dübel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 255–266. [Google Scholar]
- Kato, M.; Hanyu, Y. Colony Assay for Antibody Library Screening: Outlook and Comparison to Display Screening. In Antibody Engineering; Böldicke, T., Ed.; InTech: London, UK, 2018; pp. 1–17. [Google Scholar]
- Chanussot, C.; Bellanger, L.; Ligny-Lemaire, C.; Seguin, P.; Ménez, A.; Boulain, J.C. Engineering of a recombinant colorimetric fusion protein for immunodiagnosis of insulin. J. Immunol. Methods 1996, 197, 39–49. [Google Scholar] [CrossRef]
- Gillet, D.; Ezan, E.; Ducancel, F.; Gaillard, C.; Ardouin, T.; Istin, M.; Ménez, A.; Boulain, J.C.; Grognet, J.M. Enzyme Immunoassay Using a Rat Prolactin–Alkaline Phosphatase Recombinant Tracer. Anal. Chem. 1993, 65, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Bettsworth, F.; Monnet, C.; Watelet, B.; Battail-Poirot, N.; Gilquin, B.; Jolivet, M.; Menez, A.; Arnaud, M.; Ducancel, F. Functional characterization of two anti-estradiol antibodies as deduced from modelling and site-directed mutagenesis experiments. J. Mol. Recognit. 2001, 14, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.H.; Chevrier, D.; Boulain, J.C.; Guesdon, J.L. Recombinant single-chain Fv antibody fragment-alkaline phosphatase conjugate for one-step immunodetection in molecular hybridization. J. Immunol. Methods 1999, 227, 177–185. [Google Scholar] [CrossRef]
- Ducancel, F.; Gillet, D.; Carrier, A.; Lajeunesse, E.; Ménez, A.; Boulain, J.C. Recombinant colorimetric antibodies: Construction and characterization of a bifunctional F(ab)2/Alkaline phosphatase conjugate produced in Escherichia coli. Bio/Technology 1993, 11, 601–605. [Google Scholar] [CrossRef]
- Carrier, A.; Ducancel, F.; Settiawan, N.B.; Cattolico, L.; Maillère, B.; Léonetti, M.; Drevet, P.; Ménez, A.; Boulain, J.C. Recombinant antibody-alkaline phosphatase conjugates for diagnosis of human IgGs: Application to anti-HBsAg detection. J. Immunol. Methods 1995, 181, 177–186. [Google Scholar] [CrossRef]
- Mérienne, K.; Germain, N.; Zinn-Justin, S.; Boulain, J.C.; Ducancel, F.; Ménez, A. The functional architecture of an acetylcholine receptor-mimicking antibody. J. Biol. Chem. 1997, 272, 23775–23783. [Google Scholar] [CrossRef] [Green Version]
- Griep, R.A.; Van Twisk, C.; Kerschbaumer, R.J.; Harper, K.; Torrance, L.; Himmler, G.; Van der Wolf, J.M.; Schots, A. pSKAP/S: An expression vector for the production of single-chain Fv alkaline phosphatase fusion proteins. Protein Expr. Purif. 1999, 16, 63–69. [Google Scholar] [CrossRef]
- Martin, C.D.; Rojas, G.; Mitchell, J.N.; Vincent, K.J.; Wu, J.; McCafferty, J.; Schofield, D.J. A simple vector system to improve performance and utilisation of recombinant antibodies. BMC Biotechnol. 2006, 6, 46. [Google Scholar]
- Boulain, J.C.; Ducancel, F. Expression of recombinant alkaline phosphatase conjugates in Escherichia coli. Methods Mol. Biol. (CliftonNj) 2004, 267, 101–112. [Google Scholar]
- Dong, J.X.; Li, Z.F.; Lei, H.T.; Sun, Y.M.; Ducancel, F.; Xu, Z.L.; Boulain, J.C.; Yang, J.Y.; Shen, Y.D.; Wang, H. Development of a single-chain variable fragment-alkaline phosphatase fusion protein and a sensitive direct competitive chemiluminescent enzyme immunoassay for detection of ractopamine in pork. Anal. Chim. Acta 2012, 736, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hanyu, Y. Single-step colony assay for screening antibody libraries. J. Biotechnol. 2017, 255, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.H.; Lamoure, C.; Le Du, M.-H.; Cattolico, L.; Lajeunesse, E.; Lemaître, F.; Pearson, A.; Ducancel, F.; Ménez, A.; Boulain, J.-C. Improving Escherichia coli Alkaline Phosphatase Efficacy by Additional Mutations inside and outside the Catalytic Pocket. ChemBioChem 2001, 2, 517–523. [Google Scholar] [CrossRef]
- Skerra, A.; Dreher, M.L.; Winter, G. Filter screening of antibody Fab fragments secreted from individual bacterial colonies: Specific detection of antigen binding with a two-membrane system. Anal. Biochem. 1991, 196, 151–155. [Google Scholar] [CrossRef]
- Dreher, M.L.; Gherardi, E.; Skerra, A.; Milstein, C. Colony assays for antibody fragments expressed in bacteria. J. Immunol. Methods 1991, 139, 197–205. [Google Scholar] [CrossRef]
- Kato, M.; Hanyu, Y. Single-step colony assay with autoinduction of scFv expression for the screening of antibody libraries. BioTechniques 2019, 66, 194–197. [Google Scholar] [CrossRef]
- Schaefer, J.V.; Honegger, A.; Plückthun, A. Construction of scFv Fragments from Hybridoma or Spleen Cells by PCR Assembly. In Antibody Engineering; Kontermann, R., Dübel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–44. [Google Scholar]
- He, J.; Tao, X.; Wang, K.; Ding, G.; Li, J.; Li, Q.X.; Gee, S.J.; Hammock, B.D.; Xu, T. One-step immunoassay for the insecticide carbaryl using a chicken single-chain variable fragment (scFv) fused to alkaline phosphatase. Anal. Biochem. 2019, 572, 9–15. [Google Scholar] [CrossRef]
- Xu, Z.L.; Dong, J.X.; Wang, H.; Li, Z.F.; Beier, R.C.; Jiang, Y.M.; Lei, H.T.; Shen, Y.D.; Yang, J.Y.; Sun, Y.M. Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of o,o-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. J. Agric. Food Chem. 2012, 60, 5076–5083. [Google Scholar] [CrossRef]
- Ayyar, B.V.; Arora, S.; Ravi, S.S. Optimizing antibody expression: The nuts and bolts. Methods 2017, 116, 51–62. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of recombinant antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannoni, L.; Viti, F.; Zardi, L.; Neri, D. Isolation of anti-angiogenesis antibodies from a large combinatorial repertoire by colony filter screening. Nucleic Acids Res. 2001, 29, E27. [Google Scholar] [CrossRef] [Green Version]
- Hayhurst, A. Improved expression characteristics of single-chain Fv fragments when fused downstream of the Escherichia coli maltose-binding protein or upstream of a single immunoglobulin-constant domain. Protein Expr. Purif. 2000, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Strachan, G.; Williams, S.; Moyle, S.P.; Harris, W.J.; Porter, A.J.R. Reduced toxicity of expression, in Escherichia coli, of antipollutant antibody fragments and their use as sensitive diagnostic molecules. J. Appl. Microbiol. 1999, 87, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Schirrmann, T.; Al-Halabi, L.; Dübel, S.; Hust, M. Production systems for recombinant antibodies. Front. Biosci. A J. Virtual Libr. 2008, 13, 4576–4594. [Google Scholar] [CrossRef] [Green Version]
- Giacalone, M.J.; Gentile, A.M.; Lovitt, B.T.; Berkley, N.L.; Gunderson, C.W.; Surber, M.W. Toxic protein expression in Escherichia coli using a rhamnose-based tightly regulated and tunable promoter system. BioTechniques 2006, 40, 355–364. [Google Scholar] [CrossRef]
- Sepulveda, J.; Shoemaker, C.B. Design and testing of PCR primers for the construction of scFv libraries representing the immunoglobulin repertoire of rats. J. Immunol. Methods 2008, 332, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Kato, M.; Hanyu, Y. Construction of an scFv library by enzymatic assembly of VL and VH genes. J. Immunol. Methods 2013, 396, 15–22. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |





| Number of Colonies | Number of Positive Spots | Ratio (%) | Complete scFv Insert | |
|---|---|---|---|---|
| scFv-PhoA library | 12,102 | 224 | 1.85 | 224 |
| scFv library | 11,083 | 194 | 1.75 | 192 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanyu, Y.; Kato, M. Screening Antibody Libraries with Colony Assay Using scFv-Alkaline Phosphatase Fusion Proteins. Molecules 2020, 25, 2905. https://doi.org/10.3390/molecules25122905
Hanyu Y, Kato M. Screening Antibody Libraries with Colony Assay Using scFv-Alkaline Phosphatase Fusion Proteins. Molecules. 2020; 25(12):2905. https://doi.org/10.3390/molecules25122905
Chicago/Turabian StyleHanyu, Yoshiro, and Mieko Kato. 2020. "Screening Antibody Libraries with Colony Assay Using scFv-Alkaline Phosphatase Fusion Proteins" Molecules 25, no. 12: 2905. https://doi.org/10.3390/molecules25122905