GC-MS Composition and Olfactory Profile of Concretes from the Flowers of Four Nicotiana Species
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
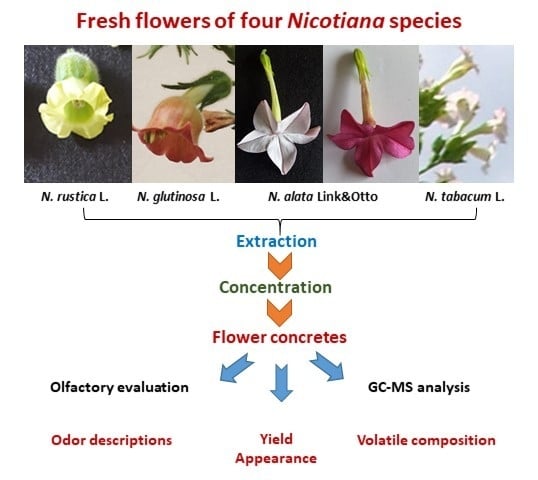
4.1. Plant Material
4.2. Obtaining of Nicotiana Flower Concretes
4.3. Olfactory Evaluation of the Concretes
4.4. GC-MS analysis of Nicotiana Flower Concretes
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knapp, S.; Chase, M.; Clarkson, J. Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). Taxon 2004, 53, 73–82. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M. Ecological roles and biological activities of specialized metabolites from the genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef] [PubMed]
- Goodspeed, T. On the evaluation of the genus Nicotiana. Proc. Natl. Acad. Sci. USA 1947, 33, 158–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chase, M.; Knapp, S.; Cox, A.; Clarkson, J.; Butsko, Y.; Joseph, J.; Savolainen, V.; Parokonny, A. Molecular systematic, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). Ann. Bot. 2003, 92, 107–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, K. Monograph of tobacco (Nicotiana tabacum). Ind. J. Drugs 2014, 2, 5–23. [Google Scholar]
- Lücker, J.; Schwab, W.; van Hautum, B.; Blaas, J.; van der Plas, L.; Bouwmeester, H.; Verhoeven, H. Increased and altered fragrance of tobacco plants after metabolic engineering using three monoterpene synthases from lemon. Plant Physiol. 2004, 134, 510–519. [Google Scholar]
- Nugroho, L.H.; Verpoorte, R. Secondary metabolism in tobacco. Plant Cell Tiss. Org. 2002, 68, 105–125. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T. The Chemical Components of Tobacco and Tobacco Smoke, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Leffingwell, J. Basic chemical constituents of tobacco leaf and differences among tobacco types. In Tobacco: Production, Chemistry and Technology; Davis, D., Nielsen, M., Eds.; Blackwell Science: London, UK, 1999; pp. 265–284. [Google Scholar]
- Loughrin, J.; Hamilton-Kemp, T.; Andersen, R.; Hildebrand, D. Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N suaveolens. Physiologia Plantarium 1991, 83, 492–496. [Google Scholar] [CrossRef]
- Loughrin, J.; Hamilton-Kemp, T.; Burton, H.; Andersen, R.; Hildebrand, D. Glycosidically bound volatile components of Nicotiana sylvestris and N suaveolens flowers. Phytochemistry 1992, 31, 1537–1540. [Google Scholar] [CrossRef]
- Raguso, R.; Levin, R.; Foose, S.; Holmberg, M.; McDade, L. Fragrance chemistry, nocturnal rhythms and pollination ‘‘syndromes’’ in Nicotiana. Phytochemistry 2003, 63, 265–284. [Google Scholar] [CrossRef]
- Zha, H.-G.; Flowers, V.L.; Yang, M.; Chen, L.-Y.; Sun, H. Acidic α-galactosidase is the most abundant nectarin in floral nectar of common tobacco (Nicotiana tabacum). Ann. Bot. 2012, 109, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piechulla, B.; Bartelt, R.; Brosemann, A.; Effmert, U.; Bouwmeester, H.; Hippauf, F.; Brandt, W. The a-terpineol to 1,8-cineole cyclization reaction of tobacco terpene synthases. Plant Physiol. 2016, 172, 2120–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguso, R.; Schlumpberger, B.; Kaczorowski, R.; Holtsford, T. Phylogenetic fragrance patterns in Nicotiana sections Alatae and Suaveolentes. Phytochemistry 2006, 67, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Braymer, H.D.; Murphy, E.L.; Chobnet, W.; Scully, N.; Wender, S.H. Methyl ethers of quercetin in tobacco flowers. J. Org. Chem. 1960, 25, 2063–2064. [Google Scholar] [CrossRef]
- Snook, M.; Chortyk, O.; Sisson, V.; Costell, C. The flower flavonols of Nicotiana species. Phytochemistry 1992, 31, 1639–1647. [Google Scholar] [CrossRef]
- Sakai, K.-I.; Shimamoto, Y. Developmental instability in leaves and flowers of Nicotiana tabacum. Genetics 1965, 51, 801–815. [Google Scholar]
- Zhu, C.; Gerjets, T.; Sandmann, G. Nicotiana glauca engineered for the production of ketocarotenoids in flowers and leaves by expressing the cyanobacterial crtO ketolase gene. Transgenic Res. 2007, 16, 813–821. [Google Scholar] [CrossRef]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Dewey, R.E.; Xie, J. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 2013, 94, 10–27. [Google Scholar] [CrossRef]
- Loughrin, J.; Hamilton-Kemp, T.; Andersen, R.; Hildebrand, D. Volatiles from flowers of Nicotiana sylvestris, N otophora and Malus × domestica: Headspace components and day/night changes in their relative concentrations. Phytochemistry 1990, 29, 2473–2477. [Google Scholar] [CrossRef]
- Fähnrich, A.; Krause, K.; Piechulla, B. Product variability of the ‘cineole cassette’ monoterpene synthases of related Nicotiana species. Mol. Plant 2011, 4, 965–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fähnrich, A.; Brosemann, A.; Teske, L.; Neumann, M. Synthesis of ‘cineole cassette’ monoterpenes in Nicotiana section Alatae: Gene isolation, expression, functional characterization and phylogenetic analysis. Plant Mol. Biol. 2012, 79, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.; Hamilton-Kemp, T.; Andersen, R.; Hildebrand, D. Headspace compounds from flowers of Nicotiana tabacum and related species. J. Agric. Food Chem. 1990, 38, 455–460. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, Y.; Li, M.; Zhao, S.; Hu, Z. Composition, antioxidative and antimicrobial activities of tobacco flower bud oil. Tob. Sci. Technol. 2015, 48, 76–80. [Google Scholar]
- Xu, C.; Zhao, S.; Li, M.; Dai, Y.D.; Tan, L.; Liu, Y. Chemical composition, antimicrobial and antioxidant activities of essential oil from flue-cured tobacco flower bud. Biotechnol. Biotechnol. Equip. 2016, 30, 1026–1030. [Google Scholar] [CrossRef] [Green Version]
- Schlotzhauer, W.; Horvat, R.; Chortyk, O.; Jackson, D. Volatile constituents from the flowers of Nicotiana longiflora. J. Essent. Oil Res. 1994, 6, 195–197. [Google Scholar] [CrossRef]
- Schlotzhauer, W.; Horvat, R.; Chortyk, O.; Nottigham, S.; Jackson, D. Comparison of the volatile flower oils of Nicotiana rustica and N forgetiana. J. Essent. Oil Res. 1995, 7, 265–269. [Google Scholar] [CrossRef]
- Duan, S.; Du, Y.; Hou, X.; Yan, N.; Dong, W.; Mao, X.; Zhang, Z. Chemical basis of the fungicidal activity of tobacco extracts against Valsa mali. Molecule 2016, 21, 1743. [Google Scholar] [CrossRef] [Green Version]
- Lay, F.T.; Brugliera, F.; Anderson, M.A. Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 2003, 131, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Fragrantica Inc, San Diego, CA, USA Tobacco Blossom White Tobacco. Available online: https://wwwfragranticacom/notes/ (accessed on 14 May 2020).
- Cosmetic Ingredient Database (CosIng) of the European Commission. Available online: https://eceuropaeu/growth/sectors/cosmetics/cosing_en (accessed on 14 May 2020).
- UNION, PEAN. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. Off. J. Eur. Union. 2009, 342, 59. [Google Scholar]
- Goossens, A. Cosmetic contact allergens. Cosmetics 2016, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics-a critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials Preparation, Properties and Uses, 4th ed.; Wiley-VCH: Weinheim, NY, USA, 2001. [Google Scholar]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Dimanov, D.; Dyulgersky, Y.; Zheliazkov, I. Selection of big leaf tobaccos in Bulgaria-status and perspectives (review). Agric. Sci. 2012, 11, 57–60. [Google Scholar]
- Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Docheva, M.; Hristeva, T.; Damyanova, S.; Nikolov, N. Chemical constituents in leaves and aroma products of Nicotiana rustica L tobacco. Int. J. Food Stud. 2020, 9, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Gochev, V.; Yonchev, Y.; Nikolov, N.; Zheljazkov, V.D. Terpenoids in the essential oil and concentrated aromatic products obtained from Nicotiana glutinosa L leaves. Molecules 2020, 25, 30. [Google Scholar] [CrossRef] [Green Version]
- Popova, V.; Ivanova, T.; Nikolova, V.; Stoyanova, A.; Docheva, M.; Hristeva, T.; Damyanova, S.; Nikolov, N. Biologically active and volatile compounds in leaves and extracts of Nicotiana alata Link & Otto from Bulgaria. J. Pharm. Sci. Res. 2017, 9, 2045–2051. [Google Scholar]
- Stoyanova, A.; Georgiev, E.; Atanasova, T.A. Handbook for Laboratory Practice in Essential Oils; Acad. Publ. House: Plovdiv, Bulgaria, 2007. [Google Scholar]
- Kapetanović, S. Results of extraction of tobacco flowers and inflorescences with volatile solvents with regard to yield and quality of essential oils. Tobacco 1989, 39, 91–102. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Jackson, D.M.; Danehower, D.A. Integrated case study: Nicotiana leaf surface components and their effects on insect pests and disease. In Plant Cuticles: An Integrated Functional Approach; Kerstiens, G., Ed.; BIOS Scientific Publishers, Ltd.: Oxford, UK, 1996; pp. 231–254. [Google Scholar]
- Bailey, J.; Vincent, G.; Burden, R. Diterpenes from Nicotiana glutinosa and their effect on fungal growth. J. Gen. Microbiol. 1974, 85, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Dudareva, N.; Pichersky, E. Floral scent metabolic pathways: Their regulation and evolution. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; Taylor & Francis Group: Boca Raton, FL, USA; London, UK, 2006; pp. 55–78. [Google Scholar]
- Roberts, D.L.; Rowland, R.L. Macrocyclic diterpenes α- and β-4,8,13-duvatriene-1,3-diols from tobacco. J. Org. Chem. 1962, 27, 3989–3995. [Google Scholar] [CrossRef]
- Chang, S.Y.; Grunwald, C. Duvatrienediols in cuticular wax of Burley tobacco leaves. J. Lipid. Res. 1976, 17, 7–11. [Google Scholar] [PubMed]
- Mashkovskii, M.D.; Babayan, E.A.; Oboimakova, A.N.; Bulaeva, V.M.; Severtseva, V.A.; Lyubimova, B.I.; Tentsova, A.I. The State Pharmacopoeia of the USSR, 11th ed.; Medicina: Moscow, Russia, 1990. [Google Scholar]
- International Organization for Standardization (ISO) Homepage. Available online: https://wwwisoorg/standard/36385html (accessed on 14 May 2020).
- Adams, R. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
Sample Availability: Not available. |


| Index | N. rustica | N. glutinosa | N. alata (WF)1 | N. alata (PF)2 | N. tabacum (OR)3 | N. tabacum (FCV)4 |
|---|---|---|---|---|---|---|
| Moisture (fresh flowers), % | 86.61 ± 0.75 5 | 81.13 ± 0.70 | 85.14 ± 0.75 | 84.64 ± 0.73 | 84.33 ± 0.73 | 84.49 ± 0.74 |
| Yield of concrete, % FW 6 | 0.21 ± 0.01 | 0.48 ± 0.01 | 0.27 ± 0.01 | 0.38 ± 0.01 | 0.57 ± 0.01 | 0.81 ± 0.01 |
| Yield of concrete, % DW 7 | 1.42 ± 0.01 a | 2.46 ± 0.02 b | 1.58 ± 0.01 a | 2.65 ± 0.02 b | 3.14 ± 0.03 c | 5.24 ± 0.05 d |
| Appearance of concretes 8 | Waxy, semi-solid masses with yellow-green color | |||||
| Species | Odor Description 1 |
|---|---|
| N. rustica | Typical green, herbaceous odor, with slight honey-like undertones |
| N. glutinosa | Green, slightly floral odor with honey-like undertones |
| N. alata (WF)2 | Floral, honey-like odor with sweet undertones |
| N. alata (PF)3 | Typical floral odor with slight green and honey-like undertones |
| N. tabacum (OR)4 | Very strong green odor with fresh undertones, reminiscent of freshly cut hay |
| N. tabacum (FCV)5 | Very strong green odor with slight floral and honey-like undertones |
| No | Compounds | RI 1 | Content, % of TIC 2 | |||||
|---|---|---|---|---|---|---|---|---|
| N. rustica | N. glutinosa | N. alata (WF)3 | N. alata (PF)4 | N. tabacum (OR)5 | N. tabacum (FCV)6 | |||
| 1 | Butanoic acid, 3-methyl- | 851 | 1.71 ± 0.01 7 | 0.25 ± 0.00 | nd | nd | 1.89 ± 0.01 | nd |
| 2 | α-3 hexene | 939 | nd 8 | nd | nd | nd | nd | 0.13 ± 0.00 |
| 3 | Pentanoic acid, 3-methyl | 946 | 11.11 ± 0.10 | 0.19 ± 0.00 | nd | nd | 12.22 ± 0.11 | nd |
| 4 | Benzaldehyde | 965 | nd | nd | nd | nd | nd | 0.29 ± 0.00 |
| 5 | β-Pinene | 979 | nd | nd | nd | nd | nd | 0.11 ± 0.00 |
| 6 | Hexanoic acid, 5-methyl- | 983 | nd | 1.24 ± 0.01 | nd | nd | nd | nd |
| 7 | Hexanoic acid, 4-methyl- | 1008 | 2.27 ± 0.02 | 2.58 ± 0.02 | nd | nd | 2.49 ± 0.02 | nd |
| 8 | Limonene | 1030 | nd | nd | nd | nd | nd | 0.51 ± 0.00 |
| 9 | 1,8-cineole | 1032 | nd | nd | nd | nd | nd | 0.15 ± 0.00 |
| 10 | Linalool | 1103 | nd | nd | nd | nd | nd | 0.17 ± 0.00 |
| 11 | n-Octanoic acid | 1173 | nd | nd | 3.81 ± 0.03 | 3.43 ± 0.03 | nd | nd |
| 12 | Naphtalene | 1181 | nd | nd | nd | nd | nd | 0.14 ± 0.00 |
| 13 | Linalyl acetate | 1259 | nd | nd | nd | nd | nd | 0.09 ± 0.00 |
| 14 | 2-Methylmaphtalene | 1295 | nd | nd | nd | nd | nd | 0.21 ± 0.00 |
| 15 | 1-Methylnaphtalene | 1312 | nd | nd | nd | nd | nd | 0.11 ± 0.00 |
| 16 | Nicotine | 1367 | 2.86 ± 0.02 | 1.63 ± 0.01 | 0.17 ± 0.00 | 0.16 ± 0.00 | 2.14 ± 0.02 | 0.31 ± 0.00 |
| 17 | Solanone | 1374 | nd | nd | nd | nd | nd | 0.48 ± 0.00 |
| 18 | β-Caryophyllene | 1419 | nd | nd | nd | nd | nd | 0.07 ± 0.00 |
| 19 | β-Farnesene | 1448 | nd | nd | nd | nd | nd | 0.08 ± 0.00 |
| 20 | n-Pentadecane | 1500 | nd | nd | nd | nd | nd | 1.55 ± 0.01 |
| 21 | n-Hexadecane | 1600 | nd | nd | nd | nd | nd | 2.41 ± 0.02 |
| 22 | 4-allyl-syringol | 1614 | nd | nd | nd | nd | nd | 1.02 ± 0.01 |
| 23 | 4-propyl syringol | 1620 | nd | nd | nd | nd | nd | 1.36 ± 0.01 |
| 24 | Syringaldehyde | 1673 | nd | nd | nd | nd | nd | 0.72 ± 0.00 |
| 25 | n-Heptadecane | 1700 | nd | nd | nd | nd | nd | 0.52 ± 0.00 |
| 26 | Tetradecanoic acid | 1778 | nd | nd | nd | nd | nd | 0.30 ± 0.00 |
| 27 | Phenanthrene | 1790 | nd | nd | nd | nd | nd | 0.17 ± 0.00 |
| 28 | Athracene | 1798 | nd | nd | nd | nd | nd | 0.11 ± 0.00 |
| 29 | n-Octadecane | 1800 | nd | nd | nd | nd | nd | 0.13 ± 0.00 |
| 30 | Pentadecanoic acid | 1875 | nd | nd | nd | nd | nd | 0.38 ± 0.00 |
| 31 | n-Nonadecane | 1900 | nd | nd | nd | nd | nd | 0.61 ± 0.00 |
| 32 | Sclareoloxide | 1906 | nd | 0.30 ± 0.00 | nd | nd | nd | nd |
| 33 | n-Hexadecanoic acid | 1979 | 1.08 ± 0.01 | 0.21 ± 0.00 | 0.14 ± 0.00 | 0.13 ± 0.00 | 1.19 ± 0.01 | nd |
| 34 | 13-Epimanool | 2055 | nd | 14.95 ± 0.13 | nd | nd | nd | nd |
| 35 | n-Eicosane | 2000 | nd | nd | nd | nd | nd | 0.74 ± 0.00 |
| 36 | Thunbergol | 2065 | nd | nd | nd | nd | nd | 2.27 ± 0.02 |
| 37 | n-Heneicosane | 2100 | 1.63 ± 0.01 | 0.36 ± 0.00 | 1.13 ± 0.01 | 1.02 ± 0.01 | 1.79 ± 0.01 | 0.30 ± 0.00 |
| 38 | Methyl octadecanoate | 2128 | nd | nd | nd | nd | nd | 0.49 ± 0.00 |
| 39 | Duvatrienediol isomer (α) | 2163 | nd | nd | nd | nd | nd | 4.86 ± 0.04 |
| 40 | (Z,Z)-Linoleic acid | 2168 | 3.39 ± 0.03 | 0.56 ± 0.00 | 0.25 ± 0.00 | 0.23 ± 0.00 | 3.03 ± 0.02 | 3.52 ± 0.03 |
| 41 | Octadecanoic acid | 2188 | nd | nd | nd | nd | nd | 0.61 ± 0.00 |
| 42 | n-Docosane | 2200 | 0.25 ± 0.00 | 0.75 ± 0.00 | 0.43 ± 0.00 | 0.39 ± 0.00 | 0.27 ± 0.00 | 1.23 ± 0.01 |
| 43 | Duvatrienediol isomer (β) | 2214 | nd | nd | nd | nd | nd | 2.01 ± 0.01 |
| 44 | Sclareol | 2222 | nd | 25.89 ± 0.24 | nd | nd | 0.24 ± 0.00 | nd |
| 45 | 4,8,13-Duvatriene-1,3-diol isomer (α) | 2275 | nd | nd | nd | nd | nd | 4.26 ± 0.03 |
| 46 | 4,8,13-Duvatriene-1,3-diol isomer (β) | 2282 | nd | nd | nd | nd | nd | 2.43 ± 0.02 |
| 47 | Tributyl acetylcitrate | 2254 | 9.10 ± 0.08 | 0.31 ± 0.00 | 0.76 ± 0.00 | 30.69 ± 0.29 | 10.01 ± 0.09 | nd |
| 48 | Podocarp-7-en-3-one, 13β-methyl-13-vinyl- | 2274 | nd | 5.65 ± 0.05 | nd | nd | nd | nd |
| 49 | 3-α-Hydroxy manool | 2286 | nd | 16.02 ± 0.15 | nd | nd | nd | nd |
| 50 | n-Tricosane | 2300 | 1.35 ± 0.01 | 0.37 ± 0.00 | 0.32 ± 0.00 | 0.29 ± 0.00 | 1.48 ± 0.01 | 8.07 ± 0.07 |
| 51 | n-Tetracosane | 2400 | 1.79 ± 0.01 | 0.48 ± 0.00 | 0.72 ± 0.00 | 0.65 ± 0.00 | 1.97 ± 0.01 | 8.28 ± 0.07 |
| 52 | n-Pentacosane | 2500 | 2.77 ± 0.02 | 0.33 ± 0.00 | 0.81 ± 0.00 | 0.73 ± 0.00 | 3.05 ± 0.02 | 12.86 ± 0.11 |
| 53 | n-Hexacosane | 2600 | 2.05 ± 0.01 | 0.46 ± 0.00 | 0.62 ± 0.00 | 0.56 ± 0.00 | 2.26 ± 0.01 | 14.13 ± 0.13 |
| 54 | n-Heptacosane | 2700 | 2.51 ± 0.02 | 0.59 ± 0.00 | 0.45 ± 0.00 | 0.40 ± 0.00 | 2.76 ± 0.02 | 5.14 ± 0.04 |
| 55 | Phthalic acid, diisooctyl ester | 2712 | 2.37 ± 0.02 | 1.03 ± 0.01 | 3.62 ± 0.03 | 3.26 ± 0.03 | 2.61 ± 0.02 | nd |
| 56 | n-Octacosane | 2800 | 5.52 ± 0.05 | 1.16 ± 0.01 | 0.82 ± 0.00 | 0.74 ± 0.00 | 5.07 ± 0.04 | 4.31 ± 0.03 |
| 57 | Terephthalic acid, di(2-ethylhexyl) ester | 2869 | nd | 9.48 ± 0.08 | 42.85 ± 0.40 | 4.56 ± 0.04 | nd | nd |
| 58 | n-Nonacosane | 2900 | 4.37 ± 0.04 | 3.49 ± 0.03 | 1.21 ± 0.01 | 4.09 ± 0.03 | 3.81 ± 0.03 | 3.36 ± 0.03 |
| 59 | n-Triacontane | 3000 | 12.07 ± 0.11 | 1.19 ± 0.01 | 6.62 ± 0.06 | 6.96 ± 0.05 | 13.28 ± 0.12 | 3.95 ± 0.03 |
| 60 | n-Hentriacontane | 3100 | 2.54 ± 0.02 | 0.90 ± 0.00 | 2.65 ± 0.02 | 4.38 ± 0.04 | 2.09 ± 0.02 | nd |
| 61 | n-Dotriacontane | 3200 | 4.31 ± 0.04 | 1.51 ± 0.01 | 11.92 ± 0.10 | 14.72 ± 0.13 | 3.15 ± 0.03 | nd |
| 62 | n-Tritriacontane | 3300 | 2.80 ± 0.02 | 0.84 ± 0.00 | 3.18 ± 0.03 | 5.86 ± 0.05 | 2.08 ± 0.02 | nd |
| 63 | n-Tetratriacontane | 3400 | 14.55 ± 0.13 | 3.49 ± 0.03 | 10.15 ± 0.09 | 9.13 ± 0.08 | 15.01 ± 0.14 | nd |
| 64 | n-Heptatriacontane | 3500 | 1.37 ± 0.01 | 1.16 ± 0.01 | 1.90 ± 0.01 | 2.71 ± 0.02 | 1.51 ± 0.01 | nd |
| 65 | n-Hexatriacontane | 3600 | 3.72 ± 0.03 | 0.45 ± 0.00 | 2.75 ± 0.02 | 2.47 ± 0.02 | 3.09 ± 0.02 | nd |
| Sum of the identified | 97.49 | 97.82 | 97.28 | 97.56 | 98.49 | 95.03 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Zheljazkov, V.D. GC-MS Composition and Olfactory Profile of Concretes from the Flowers of Four Nicotiana Species. Molecules 2020, 25, 2617. https://doi.org/10.3390/molecules25112617
Popova V, Ivanova T, Stoyanova A, Nikolova V, Hristeva T, Zheljazkov VD. GC-MS Composition and Olfactory Profile of Concretes from the Flowers of Four Nicotiana Species. Molecules. 2020; 25(11):2617. https://doi.org/10.3390/molecules25112617
Chicago/Turabian StylePopova, Venelina, Tanya Ivanova, Albena Stoyanova, Violeta Nikolova, Tsveta Hristeva, and Valtcho D. Zheljazkov. 2020. "GC-MS Composition and Olfactory Profile of Concretes from the Flowers of Four Nicotiana Species" Molecules 25, no. 11: 2617. https://doi.org/10.3390/molecules25112617