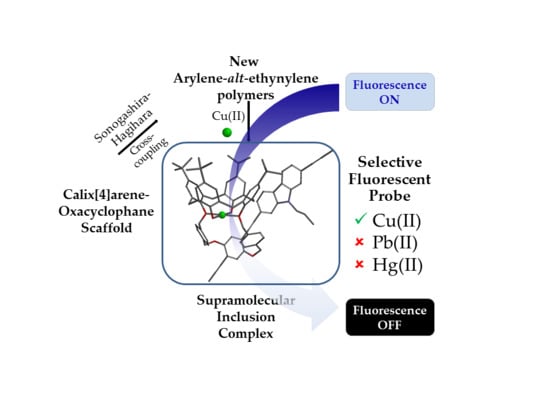
Highly Sensitive and Selective Fluorescent Probes for Cu(II) Detection Based on Calix[4]arene-Oxacyclophane Architectures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Polymeric Fluorescent Probes
2.2. Structural Characterization of Polymers
2.3. Photophysical Properties
2.4. Inclusion Complexes and Sensing of Metal Cations
2.5. Effect of Calixarene Structure on Sensing
3. Materials and Methods
3.1. Instruments and Methods
3.2. Materials
3.3. Polymers’ Synthesis
3.4. Computational Methods and Non-linear Regression Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bissell, R.A.; Prasanna de Silva, A.; Gunaratne, H.Q.N.; Lynch, P.L.M.; Maguire, G.E.M.; Sandanayake, K.R.A.S. Molecular Fluorescent Signalling with ’Fluor-Spacer-Receptor‘ Systems: Approaches to Sensing and Switching Devices via Supramolecular Photophysics. Chem. Soc. Rev. 1992, 21, 187–195. [Google Scholar] [CrossRef]
- Fabbrizzi, L.; Poggi, A. Sensors and Switches from Supramolecular Chemistry. Chem. Soc. Rev. 1995, 24, 197–202. [Google Scholar] [CrossRef]
- Prasanna de Silva, A.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 420–436. [Google Scholar] [CrossRef]
- Asfari, Z.; Böhmer, V.; Harrowfield, J.; Vicens, J. (Eds.) Calixarenes 2001; Kluwer Academic: Dordrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes—An Introduction. In Monographs in Supramolecular Chemistry; Stoddart, J.F., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Malmström, B.G.; Leckner, J. The chemical biology of copper. Curr. Opin. Chem. Biol. 1998, 2, 286–292. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Aspects Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, E.S. The 2008 WHO classification of lymphomas: Implications for clinical practice and translational research. Eur. J. Haematol. 2008, 80, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef]
- Toscano, C.D.; Guilarte, T.R. Lead neurotoxicity: From exposure to molecular effects. Brain Res. Rev. 2005, 49, 529–554. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-K.; Xu, Z.-X.; Chen, C.-F.; Huang, Z.-T. A highly efficient and selective turn-on fluorescent sensor for Cu2+ ion based on calix[4]arene bearing four iminoquinoline subunits on the upper rim. Chem. Commun 2008, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Babu, J.N.; Bhalla, V. Fluorescent chemosensor for Cu2+ ion based on iminoanthryl appended calix[4]arene. J. Inclusion Phenom. Mol. Recognit. Chem. 2010, 66, 139–145. [Google Scholar] [CrossRef]
- Chawla, H.M.; Shukla, R.; Pandey, S. Novel fluorescein appended calix[4]arenes for preferential recognition of Cu2+ ions. Tetrahedron Lett. 2013, 54, 2063–2066. [Google Scholar] [CrossRef]
- Chang, K.-C.; Luo, L.-Y.; Diau, E.W.-G.; Chung, W.-S. Highly selective fluorescent sensing of Cu2+ ion by an arylisoxazole modified calix[4]arene. Tetrahedron Lett. 2008, 49, 5013–5016. [Google Scholar] [CrossRef]
- Fischer, C.; Stapf, M.; Seichter, W.; Weber, E. Fluorescent chemosensors based on a new type of lower rim-dansylated and bridge-substituted calix[4]arenes. Supramol. Chem. 2013, 25, 371–383. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, X.; Yan, C.; Sun, J. Determination of trace copper(II) by Triton X-100 sensitized fluorescence quenching of a novel calix [4]arene Schiff base derivative. Anal. Methods 2014, 6, 575–580. [Google Scholar] [CrossRef]
- Costa, A.I.; Pinto, H.D.; Ferreira, L.F.V.; Prata, J.V. Solid-state sensory properties of Calix-poly(phenylene ethynylene)s toward nitroaromatic explosives. Sens. Actuators Chem. B 2012, 161, 702–713. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.I.; Prata, J.V. Substituted p-phenylene ethynylene trimers as fluorescent sensors for nitroaromatic explosives. Sens. Actuators Chem. B 2012, 161, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Barata, P.D.; Prata, J.V. New Entities for Sensory Chemistry based on Calix[4]arene-Carbazole Conjugates: From Synthesis to Applications. Supramol. Chem. 2013, 25, 782–797. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Cooperative Effects in the Detection of a Nitroaliphatic Liquid Explosive and an Explosive Taggant in the Vapor Phase by Calix[4]arene-Based Carbazole-Containing Conjugated Polymers. ChemPlusChem 2014, 79, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, C.M.; Costa, A.I.; Prata, J.V. A new fluorescent double-cavity calix[4]arene: Synthesis and complexation studies toward nitroanilines. Tetrahedron Lett. 2013, 54, 6602–6606. [Google Scholar] [CrossRef]
- Prata, J.V.; Costa, A.I.; Teixeira, C.M. A Solid-State Fluorescence Sensor for Nitroaromatics and Nitroanilines Based on a Conjugated Calix[4]arene Polymer. J. Fluoresc. 2020, 30, 41–50. [Google Scholar] [CrossRef]
- Prata, J.V.; Barata, P.D. Fostering protein-calixarene interactions: From molecular recognition to sensing. RSC Adv. 2016, 6, 1659–1669. [Google Scholar] [CrossRef] [Green Version]
- Prata, J.V.; Barata, P.D.; Pescitelli, G. Inherently chiral calix[4]arenes with planar chirality: Two new entries to the family. Pure Appl. Chem. 2014, 86, 1819–1828. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, C.M. New Molecular Receptors Based on Calix[4]arenes—Application to Sensorial Chemistry. Master’s Thesis, Instituto Politécnico de Lisboa, Instituto Superior de Engenharia de Lisboa, Lisboa, Portugal, 2013. Available online: http://hdl.handle.net/10400.21/3307 (accessed on 9 April 2020).
- Barata, P.D.; Costa, A.I.; Prata, J.V. Calix[4]arene-carbazole-containing polymers: Synthesis and properties. React. Funct. Polym. 2012, 72, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; p. 282. ISBN 978-0387-31278-1. [Google Scholar]
- Weert, M. van de. Fluorescence Quenching to Study Protein-ligand Binding: Common Errors. J. Fluor. 2010, 20, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- van de Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Modern Molecular Photochemistry of Organic Molecules; University Science Books: Sausalito, CA, USA, 2010; pp. 216–217. ISBN 978-1891389252. [Google Scholar]
- Liu, Y.; Han, B.-H.; Chen, Y.-T. Molecular Recognition and Complexation Thermodynamics of Dye Guest Molecules by Modified Cyclodextrins and Calixarenesulfonates. J. Phys. Chem. B 2002, 106, 4678–4687. [Google Scholar] [CrossRef]
- Brown, A.M. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Meth. Programs Biomed. 2001, 65, 191–200. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Ransnas, L.A. Fitting curves to data using nonlinear regression: A practical and nonmathematical review. FASEB J. 1987, 1, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.J.; Bühlmann, P. Getting More out of a Job Plot: Determination of Reactant to Product Stoichiometry in Cases of Displacement Reactions and n:n Complex Formation. Org. Chem. 2011, 76, 8406–8412. [Google Scholar] [CrossRef] [PubMed]
- Sanz, S.; Ferreira, K.; McIntosh, R.D.; Dalgarno, S.J.; Brechin, E.K. Calix[4]arene-supported FeIII2 LnIII2 clusters. Chem. Commun. 2011, 47, 9042–9044. [Google Scholar] [CrossRef] [PubMed]
- Karotsis, G.; Kennedy, S.; Dalgarno, S.J.; Brechin, E.K. Calixarene supported enneanuclear Cu(II) clusters. Chem. Commun. 2010, 46, 3884–3886. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Lo, R.; Chakraborty, A.; Gunupuru, R.; Maity, D.; Ganguly, B.; Paul, P. Calix[4]arene based fluorescent chemosensor bearing coumarin as fluorogenic unit: Synthesis, characterization, ion-binding property and molecular modelling. Polyhedron 2013, 50, 592–601. [Google Scholar] [CrossRef]
- Garribba, E.; Micera, G. The Determination of the Geometry of Cu(II) Complexes An EPR Spectroscopy Experiment. J. Chem. Educ. 2006, 83, 1229–1232. [Google Scholar] [CrossRef]
- Spartan’14 Molecular Modeling Program; Wavefunction, Inc.: Irvine, CA, USA, 2014.
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Crawford, K.B.; Goldfinger, M.B.; Swager, T.M. Na+ Specific Emission Changes in an Ionophoric Conjugated Polymer. J. Am. Chem. Soc. 1998, 120, 5187–5192. [Google Scholar] [CrossRef]
- Fan, L.-J.; Zhanga, Y.; Murphya, C.B.; Angell, S.E.; Parker, M.F.L.; Flynn, B.R.; Jones, W.E., Jr. Fluorescent conjugated polymer molecular wire chemosensors for transition metal ion recognition and signalling. Coord. Chem. Rev. 2009, 253, 410–422. [Google Scholar] [CrossRef]
- Sahin, O.; Yilmaz, M. Synthesis and fluorescence sensing properties of novel pyrene-armed calix[4]arene derivatives. Tetrahedron 2011, 67, 3501–3508. [Google Scholar] [CrossRef]
- Talanova, G.G.; Talanov, V.S. Dansyl-containing fluorogenic calixarenes as optical chemosensors of hazardous metal ions: A mini-review. Supramol. Chem. 2010, 22, 838–852. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Colleran, J.; Twamley, B.; Heaney, F. Highly Selective Fluorimetric Turn-Off Detection of Copper(II) by Two Different Mechanisms in Calix[4]arene-Based Chemosensors and Chemodosimeters. ChemPlusChem 2019, 84, 1610–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Sun, P.; Fan, Q.; Zhang, L.; Minami, T. Highly selective detection of copper(II) by a “ligand-free” conjugated copolymer in nucleophilic solvents. Front. Chem. Sci. Eng. 2020, 14, 105–111. [Google Scholar] [CrossRef]
- Murphy, C.B.; Zhang, Y.; Troxler, T.; Ferry, V.; Martin, J.J.; Jones, W.E. Probing Förster and Dexter Energy-Transfer Mechanisms in Fluorescent Conjugated Polymer Chemosensors. J. Phys. Chem. B 2004, 108, 1537–1543. [Google Scholar] [CrossRef]
- Higuchi, Y.; Narita, M.; Niimi, T.; Ogawa, N.; Hamada, F.; Kumagai, H.; NobuhikoIki, N.; Miyano, S.; Kabuto, C. Fluorescent Chemo-Sensor for Metal Cations Based on Thiacalix[4]arenes Modified with Dansyl Moieties at the Lower Rim. Tetrahedron 2000, 56, 4659–4666. [Google Scholar] [CrossRef]
- Ocak, Ü.; Ocak, M.; Surowiec, K.; Bartsch, R.A.; Gorbunova, M.G.; Tu, C.; Surowiec, M.A. Metal ion complexation in acetonitrile by di-ionized calix[4]arenes bearing two dansyl fluorophores. Inclusion Phenom. Macrocyclic Chem. 2009, 63, 131–139. [Google Scholar] [CrossRef]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- A Guide to Recording Fluorescence Quantum Yields, Horiba Scientific. Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Fluorescence/quantumyieldstrad.pdf (accessed on 2 March 2020).
Sample Availability: Samples of some compounds are available from the authors upon request. |








| Polymer | Yield (%) 2 | Mw (g/mol) 3 | Mn (g/mol) 3 | Mw/Mn3 |
|---|---|---|---|---|
| 4 | 58 | 14842 | 5276 | 2.81 |
| 5 | 66 | 66468 | 15587 | 4.26 |
| Polymer | λabs max/nm (logεmax) | Eg/eV 1 | λem max/nm | Stokes Shift/nm 2 | ΦF3 |
|---|---|---|---|---|---|
| 4 | 420 (4.621) | 2.87 | 431 4 458 5 | 11 | 0.65 |
| 5 | 422 (4.682) | 2.86 | 439 4 467 5 | 17 | 0.59 |
| Host | Ka/M−1 | ΔG/kJ/mol 1 |
|---|---|---|
| Polymer 4 | 5.29 × 103 | −21.2 |
| Polymer 5 | 8.52 × 104 | −28.1 |
| Polymer 6 | 5.30 × 104 | −27.0 |
| Calix-OCP-2-CBZ | 6.62 × 104 | −27.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.I.; Barata, P.D.; Fialho, C.B.; Prata, J.V. Highly Sensitive and Selective Fluorescent Probes for Cu(II) Detection Based on Calix[4]arene-Oxacyclophane Architectures. Molecules 2020, 25, 2456. https://doi.org/10.3390/molecules25102456
Costa AI, Barata PD, Fialho CB, Prata JV. Highly Sensitive and Selective Fluorescent Probes for Cu(II) Detection Based on Calix[4]arene-Oxacyclophane Architectures. Molecules. 2020; 25(10):2456. https://doi.org/10.3390/molecules25102456
Chicago/Turabian StyleCosta, Alexandra I., Patrícia D. Barata, Carina B. Fialho, and José V. Prata. 2020. "Highly Sensitive and Selective Fluorescent Probes for Cu(II) Detection Based on Calix[4]arene-Oxacyclophane Architectures" Molecules 25, no. 10: 2456. https://doi.org/10.3390/molecules25102456