Crystal Chemistry of High-Temperature Borates
Abstract
:1. Introduction
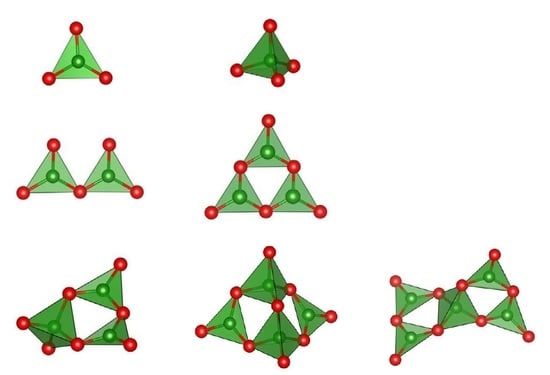
2. Hierarchy of Basic Structural Clusters
2.1. Fundamental Structural Units
2.2. Combined Structural Units
2.3. Complete Radicals of Polyanions
3. Polymerization of Boron-Oxygen Radicals
4. Polymorphism of BnOm Polyanions
5. Isostructural Series
6. Structural Formulas of Polyborates
7. Classification
- (1)
- In crystal structures each boron atom is bonded with three or with four oxygen atoms in BO3–triangles and BO4–tetrahedra;
- (2)
- In one structure not only triangular or only tetrahedral coordination is possible, but both of them jointly as well;
- (3)
- Isolated BO3–triangles and BO4–tetrahedra are not found jointly, insular polyanions;
- (4)
- A decrease in the N = NM/NB ratio (N-factor), as well as an increase in cation size (although to a smaller extent), leads to an increase in the degree of polymerization of the anion and raises the n = n∆/nt number (at N ≤ 1), whereas an increase in cation charge causes the inverse tendency;
- (5)
- Polymerization, or the formation of chains, layers and frameworks, is actualizing by the sharing corners of triangles and tetrahedra (the sharing edges has up to now not been proved conclusively);
- (6)
- In 3D and 2D polyanions (less frequently in chainlike and insular ones), BO3–triangles and BO4–tetrahedra tend to combine into comparatively compact CSU, i.e., diborate (2∆ + 2t), triborate (2∆ + 1t), pentaborate (4∆ + 1t), boroxol (3∆), ditriborate (1∆ + 2t), dipentaborate (3∆ + 2t) and other single and double ringed boron-oxygen negative charged polymerized radicals;
- (7)
- Complex polyanions of anhydrous borates of uni- and divalent metals tend to twinning;
- (8)
- In most of complex polyanions, each oxygen atom is bonded with two boron atoms, for such compounds as MxO⋅mB2O3 with m > 1, there is n = m − 1 relationship (where n = n∆/nt);
- (9)
- As an exclusion for 2D and 3D highly condensed polyanions, the coordination numbers of oxygen atoms (relative to boron) can be equal to one or three (in cubic boracite even to four).
- (a)
- Borates proper (also, there is a sense to divide this very numerous group, having diverse cations, into two subgroups: aI—monocationic or “simple” borates and all-binary and more complex compounds);
- (b)
- Borosilicates;
- (c)
- Boroaluminates;
- (d)
- Boroberyllates;
- (e)
- Borocarbonates;
- (f)
- Boromolybdates and borotungstates.
8. Structural Aspects of Acid-Base Properties
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Inorganic Crystal Structure Data Base—ICSD; Fachinformations zentrum (FIZ) Karlsruhe: Karlsruhe, Germany, 2018; https://www.crystallography.net/cod/.
- Leonyuk, N.I. Structural aspects in crystal growth of anhydrous borates. J. Cryst. Growth 1997, 174, 301–307. [Google Scholar] [CrossRef]
- Leonyuk, N.I. Growth of new optical crystals from boron-containing fluxes. Crystallogr. Rep. 2008, 53, 511–518. [Google Scholar] [CrossRef]
- Timchenko, T.I.; Leonyuk, N.I.; Pashkova, A.V.; Zhuravleva, O.N. Monophase field crystallization of NdAl-orthoborate in molybdate-based flux system. Sov. Phys. Dokl. 1979, 24, 336–337. [Google Scholar]
- Hawthorne, F.C.; Burns, P.C.; Grice, J.D. The crystal chemistry of boron. Rev. Mineral. Geochem. 1996, 33, 41–115. [Google Scholar]
- Christ, C.L.; Clark, J.R. A crystal-chemical classification of borate structures with emphasis on hydrated borates. Phys. Chem. Min. 1977, 2, 59–87. [Google Scholar] [CrossRef]
- Bondareva, O.S.; Yegorov-Tismenko, Y.K.; Simonov, M.A.; Malinovsky, Y.A.; Belov, N.V. Chrystal-chemical features of the Li, Zn, Mn and LiZn(Mn) borate structures. Russ. J. Kryst. 1980, 25, 545–554. [Google Scholar]
- Fang, S.M. The crystal structure of sodium metaborate Na3(B3O6). Z. Krist. 1938, 99, 1–8. [Google Scholar]
- Zachariasen, W.H. The crystal structure of potassium metaborate, K3(B3O6). J. Phys. Chem. 1937, 5, 919–922. [Google Scholar] [CrossRef]
- Krogh-Moe, J. Refinement of the crystal structure of lithium diborate, Li2O·2B2O3. Acta cryst. 1968, B24, 179–181. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of sodium diborate, Na2O·2B2O3. Acta Cryst. 1974, B30, 578–583. [Google Scholar] [CrossRef]
- Krogh-Moe, J. Refinement of the crystal structure of cesium triborate, Cs2O·3B2O3. Acta Cryst. 1974, 30, 1178–1179. [Google Scholar] [CrossRef]
- Krogh-Moe, J. Least-squares refinement of the crystal structure of potassium pentaborate. Acta Cryst. 1965, 18, 1088–1089. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of potassium diborate, K2O·2B2O3. Acta Cryst. 1972, B28, 3089–3093. [Google Scholar] [CrossRef]
- Zachariasen, W.H.; Ziegler, G.E. The crystal structure of calcium metaborate, CaB2O4. Z. Cryst. 1932, 83, 354–361. [Google Scholar] [CrossRef]
- Shashkin, D.P.; Simonov, M.A.; Belov, N.V. The crystal structure of calciborite CaB2O4 = Ca2[BO3BO]2. Sov. Phys Dokl. 1970, I95, 345–347. [Google Scholar]
- Marezio, M.; Plettinger, H.A.; Zachariasen, W.H. Refinement of the calcium metaborate structure. Acta Cryst. 1963, 16, 390–392. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.-G.; Cheng, W.-D.; Chen, J.-T.; Huang, J.-S.; Zhang, Q.-E. Triclinic Mg2B2O5. Acta Cryst. 1995, C51, 351–352. [Google Scholar] [CrossRef]
- Lin, Q.-S.; Cheng, W.-D.; Chen, J.-T.; Huang, J.-S. Crystal and electronic structures and linear optics of strontium pyroborate. J. Solid State Chem. 1999, 144, 30–34. [Google Scholar] [CrossRef]
- Dowty, E.; Clark, J.R. Crystal structure refinements for orthorhombic boracite, Mg3ClB7O13, and a trigonal iron-rich analogue. Z. Krist. 1973, 138, 64–99. [Google Scholar] [CrossRef]
- Marezio, M.; Remeika, J.P.; Dernier, P.D. The crystal structure of the high pressure phase CaB2O4 (III). Acta Cryst. 1969, B25, 955–964. [Google Scholar] [CrossRef]
- Dernier, P.D. Crystal data of two high pressure phases of SrB2O4. Acta Cryst. 1969, B25, 1001–1003. [Google Scholar] [CrossRef]
- Perloff, A.; Block, S. The crystal structure of the strontium and lead tetraborates. Acta Cryst. 1966, 20, 274–279. [Google Scholar] [CrossRef]
- Marezio, M.; Remeika, J.P.; Dernier, P.D. The crystal structure of the high pressure phase CaB2O4 (IV) and polymorphism in CaB2O4. Acta Cryst. 1969, B25, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Garcia-Blanco, S.; Rivoir, L. The crystal structure of anhydrous zinc metaborate Zn4O(BO2)6. Z. Krist. 1964, 119, 375–383. [Google Scholar] [CrossRef]
- Martinez-Pipoll, M.; Martinez-Carrera, S.; Carcia-Blanco, S. The crystal structure of copper metaborate CuB2O4. Acta Cryst. 1971, B27, 677–681. [Google Scholar] [CrossRef]
- Emme, H.; Despotopoulou, C.; Huppertz, H. High-pressure synthesis and crystal structure of the structurally new orthorhombic rare earth meta-oxoboratesγ-RE(BO2)3 (RE=La-Nd). Z. Anorg. Allgem. Chem. 2004, 630, 2450–2457. [Google Scholar] [CrossRef]
- Ren, M.; Lin, J.H.; Dong, Y.; Yang, L.Q.; Su, M.Z.; You, L.P. Structure and phase transition of GdBO3. Chem. Mater. 1999, 11, 1576–1580. [Google Scholar] [CrossRef]
- Cousson, A.; Gasperin, M. Synthèse et structure du borate de thorium: ThB2O5. Acta Cryst. 1991, C47, 10–12. [Google Scholar] [CrossRef]
- Hao, Y. New Insight into the Crystal Chemistry of Uranium and Thorium Borates, Borophosphates and Borate-Phosphates. Master’s Thesis, RWTH Aachen University, Aachen, Germany, 2018. [Google Scholar] [CrossRef]
- Hinteregger, E.; Hofer, T.S.; Heymann, G.; Perfler, L.; Kraus, F.; Huppertz, H. High-pressure synthesis and characterization of new actinide borates AnB4O8 (An.=Th,U). Chem. Eur. J. 2013, 19, 15985–15992. [Google Scholar] [CrossRef] [Green Version]
- Demartin, F.; Diella, V.; Gramaccioli, C.; Pezzota, F. Schiavinatoite, (Nb,Ta)BO4, the Nb analogue of behierite. Eur. J. Mineral. 2001, 13, 159–165. [Google Scholar] [CrossRef]
- Callegari, A.; Mazzi, F.; Tadini, C. Modular aspects of the crystal structures of kurchatovite and clinokurchatovite. Eur. J. Mineral. 2003, 15, 277–282. [Google Scholar] [CrossRef]
- Kadiyski, M.; Armbruster, T.; Günter, D.; Reusser, E.; Peretti, A. Johachidolite, CaAl[B3O7], a mineralogical and structural peculiarity. Eur. J. Miner. 2008, 20, 965–973. [Google Scholar] [CrossRef]
- Capitelli, F.; Chita, G.; Leonyuk, N.I.; Koporulina, E.V.; Bellatreccia, F.; Della Ventura, G. REEAl2.07(B4O10)O0.60 dimetaborates (REE = La, Pr); synthesis and X-ray structural characterization. Zeit. Kristall. 2011, 226, 219–225. [Google Scholar] [CrossRef]
- Abdullaev, G.K.; Mamedov, K.S.; Dzhafarov, G.G. Crystal structure of LaCo(BO2)5. J. Structural Chemistry. 1975, 16, 61–65. [Google Scholar] [CrossRef]
- Bondareva, O.S.; Malinovsky, Y.A.; Belov, N.V. Crystal structure of Na2Zn2MnB4.67O11. Russ. J. Kryst. 1980, 25, 944–949. [Google Scholar]
- Pashkova, A.V.; Sorokina, O.V.; Leonyuk, N.I.; Timchenko, T.I. New series of double rare-earth aluminium metaborates. Sov. Phys. Dokl. 1981, 26, 457–459. [Google Scholar]
- Sokolova, Y.V.; Azizov, A.V.; Simonov, M.A.; Leonyuk, N.I.; Belov, N.V. The crystal structure of synthetic ortho-3-borate, A15(BO3)O6. Sov. Phys. Dokl. 1978, 23, 814–816. [Google Scholar]
- Guo, S.; Liu, L.; Xia, M.; Kang, L.; Huang, Q.; Li, C.; Wang, X.; Lin, Z.; Chen, C. Be2BO3F: A phase of beryllium fluoride borate derived from KBe2BO3F2 with short UV absorption edge. Inorg. Chem. 2016, 55, 6586–6591. [Google Scholar] [CrossRef]
- He, M.; Chen, X.; Gramlich, V.; Baerlocher, C.; Zhou, T.; Hu, B. Synthesis, structure, and thermal stability of Li3AlB2O6. J. Solid State Chem. 2002, 163, 369–376. [Google Scholar] [CrossRef]
- Schuckmann, W. Zur Struktur des Calcium-Aluminium-Borates, CaAl[O/BO3]. Neues Jb. Min. Mh. 1968, 1968, 80–86. [Google Scholar]
- Nagai, T.; Ihara, M. The crystal structure of di-strontium boro-aluminate, 2SrO·Al2O3·B2O3. Yogyo-Kyokai-Shi. 1972, 80, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Kuz’micheva, G.M.; Rybakov, V.B.; Kutovoi, S.A.; Panyutin, V.L.; Oleinik, A.Y.; Plashkarev, O.G. Preparation, Structure, and Properties of New Laser Crystals Y2SiBe2O7 and Y2Al(BeB)O7. Inorganic Mater. 2002, 38, 60–65. [Google Scholar] [CrossRef]
- Samygina, V.R.; Genkona, E.A.; Maksimov, B.A.; Leonyuk, N.I. Synthesis and crystal structure of La-stillwellite. Crystallogr. Rep. 1993, 38, 744–746. [Google Scholar]
- Johansson, G. A refinement of the crystal structure of danburite. Acta cryst. 1959, 12, 522–526. [Google Scholar] [CrossRef]
- Block, S.; Perloff, A. The crystal structure of barium tetraborate, BaO·2B2O3. Acta Cryst. 1965, 19, 297–300. [Google Scholar] [CrossRef]
- Krogh-Moe, J.; Ihara, M. On the crystal structure of barium tetraborate, BaO·4B2O3. Acta Cryst. 1969, B25, 2153–2154. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of α-sodium triborate, α-Na2O·3B2O3. Acta Cryst. 1974, B30, 747–752. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of a sodium triborate modification, β-Na2O·3B2O3. Acta Cryst. 1972, B28, 1571–1576. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Shepelev, J.F.; Sennova, N.A.; Filatov, S.K. Thermal behaviour of the rigid boron-oxygen groups in the α-Na2B8O13 crystal structure. Zeit. Kristall. 2002, 217, 444–450. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of pentapotassium enneakaidekaborate, 5K2O·19B2O3. Acta Cryst. 1974, B30, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Haworth, R.; Wright, A.C.; Sinclair, R.N.; Knight, K.S.; Vedishcheva, N.M.; Polyakova, I.G.; Shakhmatkin, B.A. The polymorphs of crystalline cesium enneaborate. Eur. J.Glass Sci. Tech. 2006, B47, 352–356. [Google Scholar]
- Hyman, A.; Perloff, A.; Mauer, F.; Block, S. The crystal structure of sodium tetraborate. Acta cryst. 1967, 22, 815–820. [Google Scholar] [CrossRef]
- Jeitschko, W.; Bither, T.A.; Bierstedt, P.E. Crystal structure and ionic conductivity of Li boracites. Acta Cryst. 1977, B33, 2767–2775. [Google Scholar] [CrossRef]
- Sueno, S.; Clark, J.R.; Papike, J.J.; Konnert, J.A. Crystal structure refinement of cubic boracite. Amer. Miner. 1973, 58, 691–697. [Google Scholar]
- Zayakina, N.V.; Brovkin, A.A. Crystal structure of Ca2B6O11. Sov. Phys. Cryst. 1976, 21, 277–279. [Google Scholar]
- Zayakina, N.V.; Brovkin, A.A. Crystal structure of CaB4O7. Sov. Phys. Cryst. 1977, 22, 156. [Google Scholar]
- Chang, K.-S.; Keszler, D.A. CaAl2(BO3)2O: Crystal structure. Mat.Res.Bull. 1998, 33, 299–304. [Google Scholar] [CrossRef]
- Abdullaev, G.K.; Mamedov, K.S. The refinement of the crystal structure of lithium aluminoborate Li6Al2(BO3)4. Sov. Phys. Cryst. 1982, 27, 229–230. [Google Scholar]
- Stewner, F. Die Kristallstructur von α-Li3BO3. Acta cryst. 1971, B27, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Zachariasen, W.H. The crystal structure of lithium metaborate. Acta Cryst. 1964, 17, 749–751. [Google Scholar] [CrossRef]
- Schneider, W.; Carpenter, G.B. Bond length and thermal parameters of potassium metaborate, K3B3O6. Acta Cryst. 1970, B26, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Krogh-Moe, J. The crystal structure of cesium triborate, Cs2O·3B2O3. Acta Cryst. 1960, 13, 889–892. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of silver tetraborate, AgO·4B2O3. Acta Cryst. 1965, 18, 77–81. [Google Scholar] [CrossRef]
- Brunton, G. Refinement of the structure of NaBF4. Acta Cryst. 1968, B24, 1703–1704. [Google Scholar] [CrossRef]
- Hoard, J.L.; Blair, V. The crystal structures of rubidium and ammonium fluoborates. Amer Chem. Soc. 1935, 57, 1985–1987. [Google Scholar] [CrossRef]
- Eimerl, D.; Davis, L.; Velsko, S.; Graham, E.K.; Zalkin, A. Optical, mechanical, and thermal properties of barium borate. J. Appl. Phys. 1987, 62, 1968–1983. [Google Scholar] [CrossRef]
- White, J.G.; Miller, A.; Nielsen, R.E. Fe3BO6, a borate isostructural with the mineral norbergite. Acta Cryst. 1965, 19, 1060–1061. [Google Scholar] [CrossRef]
- Hayward, C.L.; Angel, R.J.; Ross, N.L. The structural redetermination and crystal chemistry of sinhalite, MgAlBO4. Eur. J. Mineral. 1994, 6, 313–321. [Google Scholar] [CrossRef]
- Abdullaev, G.K. Physico-chemical study and crystal chemistry of borates of groups I-III in the Periodic system. Ph.D. Thesis, Institute of Inorganic and Physical Chemistry, Baku, Azerbaijan, 1978. [Google Scholar]
- Stephenson, D.A.; Moore, P.B. The crystal structure of grandidierite (Mg,Fe)Al3SiBO9. Acta Cryst. 1968, B24, 1518–1522. [Google Scholar] [CrossRef]
- Shen, J.; Moore, P.B. Crystal structure of cappelenite, Ba(Y,RE)6[Si3B6O24]F2: A silicoborate sheet structure. Amer. Miner. 1984, 69, 190–195. [Google Scholar]
- Marezio, M.; Remeika, J.P. Polymorphism of LiMO2 compounds and high-pressure single-crystal synthesis of LiBO2. J. Chem. Phys. 1966, 44, 3348–3353. [Google Scholar] [CrossRef]
- Lei, L.; He, D.; He, K.; Qin, J.; Wang, S. Pressure-induced coordination changes in LiBO2. J. Solid State Chem. 2009, 182, 3041–3048. [Google Scholar] [CrossRef]
- Block, S.; Perloff, A.; Weir, C.E. The crystallography of some M2+ borates. Acta Cryst. 1964, 17, 314–315. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Watanabe, T.; Ito, T. The crystal structures of warwickite, ludwigite and pinakiolite. Actacryst 1950, 3, 98–107. [Google Scholar] [CrossRef]
- Moore, P.B.; Araki, T. Pinokialite M2Mn3+O2[BO3], warwickite Mg(Mg0.5,Ti0.5)O[BO3], wightmatite Mg5O(OH)5[BO3]*nH2O: Crystal chemistry of complex 3Å wallpaper structures. Amer. Min. 1974, 59, 985–1004. [Google Scholar]
- Konnert, J.A.; Appleman, D.E.; Clark, J.R.; Finger, L.W.; Kato, T.; Miúra, Y. Crystal structure and cation distribution of hulsite, a tin-iron borate. Amer. Min. 1976, 61, 116–122. [Google Scholar]
- Ehrenberg, H.; Ramdohr, P.N. Die Struktur des Nordenskiöldins. Neuesjb. Min. Beirl. 1934, B69, 1–5. [Google Scholar]
- Abrahams, S.C.; Bernstein, J.L.; Keve, E.T. Application of normal probability plot analysis to lutetium orthoborate, structure factors and parameters. J. Appl. Cryst. 1971, 4, 284–290. [Google Scholar] [CrossRef]
- Vegas, A.; Cano, F.H.; Garcia-Blanco, S. Refinement of aluminiumorthoborate. Acta cryst. 1977, B33, 3607–3609. [Google Scholar] [CrossRef]
- Capponi, J.J.; Chenavas, J.; Joubert, J.C. Nouveaux borates dàluminiumet de gallium obtenus par synthesehydrothermale a haute pression. Rull. Soc. Fr. Miner. Cristal. 1972, 95, 412–417. [Google Scholar]
- Pambaner, H.; Kindermann, B. Darstellung und Kristallographische Daten von Ortoboraten (Se)2Ca3(BO3)4. Z. Krist. 1978, 147, 63–74. [Google Scholar]
- Hong, H.Y.-P.; Dwight, K. Crystal structure and fluorescence lifetime of NdAl3(BO3)4, a promising laser material. Mater. Res. Bull. 1974, 9, 1661–1665. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Alshinskaya, L.I.; Simonov, M.A.; Leonyuk, N.I.; Timchenko, T.I.; Belov, N.V. The crystal structure of NdGa3(BO3)4. J. Struct. Chem. 1978, 19, 332–334. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Alshinskaya, L.I.; Simonov, M.A.; Leonyuk, N.I.; Timchenko, T.I.; Belov, N.V. The crystal structure of (Nd,Bi)Fe3(BO3)4. J. Struct. Chem. 1979, 20, 461–463. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Azizov, A.V.; Leonyuk, N.I.; Simonov, M.A.; Belov, N.V. YAl3(BO3)4 crystal structure. J. Struct. Chem. 1981, 22, 476–477. [Google Scholar] [CrossRef]
- Belokoneva, E.L.; Timchenko, T.I. Polytype relationships in structures of RAl3(BO3)4 borates with R=Y, Nd, Gd. Sov.Phys. Crystallogr. 1983, 28, 658–660. [Google Scholar]
- Leonyuk, N.I.; Leonyuk, L.I. Growth and characterization of RM3(BO3)4 crystals. Progr. Cryst. Growth Charact. 1995, 31, 179–278. [Google Scholar] [CrossRef]
- Bokiy, G.B.; Kravchenko, V.B. Crystallochemical classification of borates. Zh. Struct. Khimii 1966, 7, 920–937. [Google Scholar]
- Hermans, P.H. Uber die Konstitution der Borsűeren und einiger ihrer Derivate. Z. Anorg. Algem. Chem. 1925, 142, 83–110. [Google Scholar] [CrossRef]
- Tennyson, C. Eine Systematic der Borate auf Kristallchemischer Grundlage. Fortschr. Mineral. 1963, 41, 64–91. [Google Scholar]
- Ito, T.; Marimoto, N.; Sadanaga, R. The crystal structure of boracite. Acta Cryst. 1951, 4, 310–316. [Google Scholar] [CrossRef]
- Krogh-Moe, J. The crystal structure of strontium diborate, SrO·2B2O3. Acta Cryst. 1964, 18, 2055–2060. [Google Scholar] [CrossRef] [Green Version]
- Heller, G. Darstellung und Systematisierung von Boraten und Polyboraten. Chem. Forsch. 1970, 15, 206–280. [Google Scholar]
- Leonyuk, N.I. Classification of anhydrous borates. Mosc. Univ. Geol. Bull. 1983, 38, 29–36. [Google Scholar]
- Rollet, A.P.; Bouaziz, R. Le systeme binaire oxide lithium anhydride borique. Compt. Rend. De L’acad Sci. 1955, 240, 2417–2419. [Google Scholar]
- Flood, H.; Forland, T. The Acidic and Basic Properties of Oxides. Acta Chem. Scand. 1947, 1, 592–604. [Google Scholar] [CrossRef]
- Emsley, J. The Elements; Clarendon Press: Oxford, UK, 1989. [Google Scholar]












| Type of Metaborate Anion | Composition of [BO2]n Polyanion | Compound |
|---|---|---|
| Isolated rings | [B3∆O6] = [B3O6] | α-Na2O·B2O3 K2O·B2O3 Rb2O·B2O3 Cs2O·B2O3 β-BaO·B2O3 |
| Chain (one-dimensional, 1D) | [B2∆O4]1D = [B2O4]1D | α-Li2O·B2O3 CaO·B2O3 (I) SrO·B2O3 (I) |
| [(B∆O2)2B2tO4]1D = [B4O8]1D | Calciborite CaO·B2O3 (II) | |
| [(B∆O2)4BtO2]1D = [B6O12]1D | Ln2O3·3B2O3 (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb) | |
| [(B∆O2)2Al2tO4](1D) = [B2Al2O8]1D | 2CaO·Al2O3 B2O3 | |
| [(BO2)2Al2tO4]1D = [B2Al2O8]1D | 2SrO·B2O3·Al2O3 | |
| [B3tO6](1D) = [B3O6]1D (exc. Si) | Stillwellite Ce2O3·B2O3·2SiO2 | |
| [B2∆O4](D) = [B2O4]1D (exc. Mo, W) | La2O3·B2O3·2MoO3 La2O3·B2O3·2WO3 | |
| Ribbon (one-dimensional, 1D) | [(B∆O2)4(AltO2)2]1D = [B4Al2O12]1D [(B∆O2)4(GatO2)2]1D = [B4Ga2O12]1D | 3Li2O·Al2O3·2B2O3 3Li2O·Ga2O3·2B2O3 |
| Layer (two-dimensional, 2D) | [(B∆O2)4B6tO12]2D = [B10O20]2D | Ln2O3·2CoO·5B2O3 (Ln = La-Nd, Sm-Ho) |
| Framework (three-dimensional, 3D) | [BtO2]3D = [BO2]3D | γ-Li2O·B2O3 |
| [B3tO6]3D = [B3O6]3D | CaO·B2O3 (II) SrO·B2O3 (II) | |
| [B3tO6]3D = [B3O6]3D | CuO·B2O3 | |
| [B6tO12]3D = [B6O12]3D | 4ZnO·3B2O3 | |
| [(B6∆O2.5)(B∆O1.5)B4O8]3D = [B6O12]3D | CaO·B2O3 (III) SrO·B2O3 (III) | |
| [(B2tO4)(Si2O4)](3D) = [Si2B2O8]3D | Danburite—CaO·B2O3·2SiO2 |
| Type of polyanion | Composition of B4O7 Based Polyanion | Compound |
|---|---|---|
| Layers (two-dimensional, 2D) | [(B2∆BtO5.5)(B3∆B2tO8.5)]2D = [B8O14]2D | α-Na2O·2B2O3 |
| Frameworks (three- dimensional, 3D) | [B2∆B2tO7]3D = [B4O7]3D | LiO·2B2O3 |
| [B2∆B2tO7]2(3D) = [B4O7]2(3D) | MgO·2B2O3, MnO·2B2O3, ZnO·2B2O3, CdO·2B2O3 | |
| [(B2∆B2tO7)(B∆B2tO5.5)(B∆O1.5)]3D = [B8O14]3D | K2O·2B2O3 | |
| [(B2∆B2tO7)(B2∆BtO5)(BtO2)]3D = [B8O14]3D | CaO·2B2O3 | |
| [B4tO7]3D = [B4O7]3D | SrO·2B2O3, PbO·2B2O3 | |
| [(B3∆B2tO8.5)(B∆B2tO5.5)]3D = [B8O14]3D | BaO·2B2O3 |
| Radical | Type of Polyanion | Composition B3O5 and B8O13 Based Polyanions | Compound |
|---|---|---|---|
| B3O5 | Layers (two-dimensional, 2D) | [(B4∆BtO8)(B2∆BtO5)(BtO2)]2(2D) = [B9O15]2(2D) | β-Na2O·3B2O3 |
| Frameworks (three-dimensional,3D) | [(B4∆BtO8)(B2∆B2tO7)]2(3D) = [B9O15]2(3D) | α-Na2O·3B2O3 | |
| [B2∆BtO5]3D = [B3O5]3D | Cs2O·3B2O3 | ||
| B8O13 | Frameworks (three-dimensional, 3D) | [(B4∆BtO8)(B2∆BtO5)]2(3D) = [B8O13]2(3D) | α-Na2O·4B2O3 |
| [(B4∆BtO8)(B2∆BtO5)]2(3D) = [B8O13]2(3D) | 0.6Ag2O·0.4Na2O·4B2O3; BaO·4B2O3 |
| Formula in Oxides (Bulk Composition) | Structural Formula | ||
|---|---|---|---|
| Complete | Abbreviated | ||
| α-Li2O·B2O3 | α-Li2[B∆2O4]1D | α-Li2[B△2O4]1D | α-Li2[B2O4] |
| γ-Li2O·B2O3 | γ-Li[BtO2]3D | γ-Li[BtO2]3D | γ-Li[BO2] |
| γ-3Li2O·7B2O3·2LiCl | γ-Li4Cl[(B∆3Bt3O10.5)(B∆O1.5)]3D | γ-Li4Cl[B△4Bt3O12]3D | γ-Li4Cl[B7O12] |
| Li2O·2B2O3 | Li2[(B∆2Bt2O7)]3D | Li2[B△2Bt2O7]3D | Li2[B4O7] |
| MI2O·B2O3 (MI = Na-Cs) | MI3[(B∆3O6)] | MI3[B△3O6] | MI3[B3O6] |
| α-Na2O·2B2O3 | α-Na4[(B∆2BtO5.5)(B∆3Bt2O8.5)]2D | α-Na4[B△5Bt3O14]2D | α-Na4[B8O14] |
| α-Na2O·3B2O3 | α-Na6[(B2Bt2O7)(B∆4BtO8)]2(3D) | α-Na6[B△6Bt3O15]2(3D) | α-Na6[B9O15]2 |
| β-Na2O·3B2O3 | β-Na6[(B∆2BtO5)(B∆4BtO8)(BtO2)]2(2D) | β-Na6[B△6Bt3O15]2(2D) | β-Na6[B9O15]2 |
| α-Na2O·4B2O3 | α-Na4[(B∆2BtO5)(B∆4BtO8)]2(3D) | α-Na4[B△6Bt2O13]2(3D) | α-Na4[B8O13]2 |
| K2O·2B2O3 | K4[(B∆Bt2O5.5)(B∆2Bt2O7)(B∆O1.5)]3D | K4[B△4Bt4O14]3D | K4[B8O14] |
| 5K2O·19B2O3 | K2.5[(B∆2BtO5)(B∆4BtO8)(B∆O1.5)(Bt0.5O)]3D | K5[B△14Bt5O31]3D | K5[B19O31] |
| α-K2O·5B2O3 | α-K2[(B∆4BtO8)]2(3D) | α-K2[B△4BtO8]2(3D) | α-K2[B5O8]2 |
| β-MI2O·5B2O3 (MI = K, Rb) | β-MI2[(B∆4BtO8)]2(3D) | β-MI2[B△4BtO8]2(3D) | β-MI2[B5O8]2 |
| Cs2O·3B2O3 | Cs[(B∆2BtO5)]3D | Cs[B△2BtO5]3D | Cs[B3O5] |
| Cs2O·9B2O3 | Cs2[(B∆3O4.5)2(B∆2BtO5)]2(3D) | Cs2[B△8BtO14]2(3D) | Cs2[B9O14]2 |
| 0.6Ag2O·0.4Na2O·4B2O3 | Ag2.4Na1.6[(B∆2BtO5)(B∆4BtO8)]2(3D) | Ag2.4Na1.6[B△6Bt2O13]2(3D) | Ag2.4Na1.6[B8O13]2 |
| 5MIIO·7B2O3·MIIAI2—rhomb. and trig. Boracites (MII = Mg, Mn, Zn, Cd, Co, Ni, Cu; AI = Cl, NO3)—rhomb. (MII = Mg, Mn, Zn, Fe, Co, Ni, Cu; AI = F, Cl)—trig. | M3IIAI[(B∆O1.5)2(Bt6O11.5)]3D | M3IIAI[B△Bt6O13]3D | M3IIAI[B7O13] |
| 5MIIO·7B2O3·MIIAI2—cub. Boracites (MII = Mg, Mn, Fe, Ni, Co, Cu; AI = Cl, Br, NO3) | M3IIAI[(Bt7O13)]3D | M3IIAI[Bt7O13]3D | M3IIAI[B7O13] |
| MIIO·2B2O3 (MII = Mg, Mn, Zn, Cd) | M2II[(B∆2Bt2O7)]2(3D) | M2II[B∆2Bt2O7)]2(3D) | M2II[B4O7)]2 |
| MIIO·B2O3-I (MII = Ca, Sr) | MII[(B∆2O4)]1D | MII[B∆2O4]1D | MII[B2O4] |
| CaO·B2O3-II (calciborate) | Ca2[(B∆O2)2Bt2O4)]1D | Ca2[B∆2Bt2O4]1D | Ca2[B4O8] |
| MIIO·B2O3-III (MII = Ca, Sr) | M3II[(B∆O1.5)(B∆O2)(BtO2)(Bt3O6)]3D | M3II[B∆2Bt4O12]3D | M3II[(B6O12)] |
| MIIO·B2O3-IV (MII = Ca, Sr) | M3II[(Bt3O6)2]3D | M3II[(Bt3O6)2]3D | M3II[(Bt3O6)2] |
| 2CaO·3B2O3 | Ca2[(B∆2Bt3O9)2(BtO2)]3D | Ca2[B∆2Bt4O11]3D | Ca2[B6O11] |
| CaO·2B2O3-II | Ca2[(B∆2BtO5)(B∆2Bt2O7)(BtO2)]3D | Ca2[B∆4Bt4O14]3D | Ca2[B8O14] |
| MIIO·2B2O3 (MII = Sr, Po) | MII[(Bt4O7)]3D | MII[Bt4O7]3D | MII[B4O7] |
| β-BaO·B2O3 | Ba3[(B∆3O6)2] | Ba3[(B∆3O6)2] | Ba3[(B3O6)2] |
| BaO·2B2O3 | Ba2[(B∆Bt2O5.5)(B∆3Bt2O8.5)]3D | Ba2[B∆4Bt4O14]3D | Ba2[B8O14] |
| BaO·4B2O3 | Ba2[(B∆2BtO5)(B∆4BtO8)]2(3D) | Ba2[B∆6Bt2O13]2(3D) | Ba2[B8O13]2 |
| 4ZnO·3B2O3 | Zn4O[(Bt6O12)]3D | Zn4O[Bt6O12]3D | Zn4O[B6O12] |
| CuO·B2O3 | Cu3[(Bt3O6)2]3D | Cu3[(Bt3O6)2]3D | Cu3[(B3O6)2] |
| 5Al2O3·B2O3 | Al2[(Alt3O6)(B∆O3)]3D | Al2[Alt3B∆O9]3D | Al2[Al3BO9] |
| R2O3·3B2O3 (R = La-Tb) | R2[(B∆2Bt2O8)(B∆O2)2]1D | R2[B∆4Bt2O12]1D | R2[B6O12] |
| By the Composition of Anion Formers | By Cation Valency | By Cation Type (and Size) | By the Value of N Factor (N = NM/NB) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N > 1 | N = 1 | n = n∆/nt = m − 1, where m—coeff. from MxO·mB2O3 * | |||||||
| 1 < N ≤ 1/2 | 1/2 < N ≤1/3 | N < 1/3 | |||||||
| Borates | Monocationic (simple) | Monovalent | a | Li ↓ Cs | Orthoborates with isolated BO3 triangles | Metaborates, insular and chainlike ** | Polyborates, 3D and seldom - layered | Polyborates, 3D and seldom - layered | Polyborates, 3D |
| b | Ag Tl | Same | 0 | 0 | 0 | Same | |||
| Divalent | a | Be ↓ Ba | Same | Pyroborates | Metaborates, insular and chainlike** | Polyborates, 3D | Same | ||
| b | Zn, Cd, Mn, Fe, Co, Ni, Cu, Pb | Same | Same | Metaborates, 3D | Same | Same | |||
| Trivalent | a | Al | Orthoborates with BO4 tetrahedra | Orthoborates with BO3 triangles | - | - | - | ||
| b | Sc, Ti, V, Cr, Ga, In | - | Same | - | - | - | |||
| c | Fe | Orthoborates with BO4 tetrahedra | Same | - | - | - | |||
| d | Y, La-Nd, Sm-Yb | 0 | Same | - | Metaborates, chainlike | - | |||
| e | Bi | Orthoborates with BO3 triangles | - | - | 0 | 0 | |||
| Tetravalent | Th | - | - | 0 | - | - | |||
| Pentavalent | P, As, Ta, Nb | - | Orthoborates with BO3 triangles | - | - | - | |||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Borates | Binary and more complex | M+M2+ | Orthoborates with BO3 triangles | - | - | - | - | ||
| M+M3+ | Same | 0 | 0 | - | - | ||||
| M+M5+ | Same | Pyroborates | - | - | - | ||||
| M2+M2+ | - | Same | - | - | - | ||||
| M2+M3+ | Orthoborates with BO3 triangles and BO4 tetrahedra | - | Metaborates, layered (t) | Metaborates, layered (∆ + t) | - | ||||
| M2+M4+ | - | Orthoborates with BO3 triangles | - | - | - | ||||
| M3+M3+ | - | Orthoborates with BO3 triangles | Metaborates with B tetrahedrons | - | - | ||||
| Boron silicates | M2+M3+, M3+, M2+ | Orthocompounds | Metacompounds, chainlike | - | - | Polycompounds, 3D | |||
| Boron aluminates | M2+, M3+ | - | Same | Polycompounds, layered and 3D | - | - | |||
| Boron beryllates | M+ | - | - | Polycompounds, layered | Polycompounds, layered and 3D | - | |||
| Boron carbonates | M2+M3+ | Orthocompounds | - | - | - | - | |||
| Boron molybdates and boron tungstanates | M M3+ | - | - | Metacompounds, chainlike | - | - | |||
| I. Borates of Monovalent Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Cations | ||||||||
| Li | Na (Ag) | K | Rb | Cs | Tl | ||||
| Orthoborates (with isolated B triangles) | 5 | - | 0 | - | - | - | - | ||
| 3 | α-Li3[BO3] β from – 0 | 0 | - | - | - | Tl3[BO3] | |||
| 5/2 | - | 0 | - | - | - | - | |||
| 2 | - | 0 | - | - | - | - | |||
| 3/2 | 0 | 0 | - | - | - | - | |||
| Fluoroborates (with isol. BF4 tetr.) | 1 | - | Na[BF4] | K[BF4] | Rb[BF4] | Cs[BF4] | Tl[BF4] | ||
| Metaborates (ins. with boroxol gr.) * | 1 | α-Li2[B2O4]1D γ-Li[BO2]3D | α-Na3[B3O6] β phase – 0 | K3[B3O6] | α from - 0 | Cs3[B3O6] | 0 | ||
| Polyborates (skeletal, sometimes compounds, layered with diborate – D, triborate – T, pentaborate – P, boroxol – B, ditriborate – DT, dipentaborate – DP rings and additional B triangles and tetrahedrons; n = n∆/nt = m − 1) | 2/3 | - | 0 | - | - | - | - | ||
| 4/7 | α and β Cl, Br and I “boracites” – 0 γ-Li4Cl[B7O12]3D | - | - | - | - | - | |||
| 1/2 | Li4[B4O7]2(3D)2(D) | (α)-Na4[B8O14]2D ** (T+DP) β and γ phase - 0 | K4[B8O14]3D | 0 | 0 | 0 | |||
| 2/5 | 0 | 0 | - | - | - | - | |||
| 1/3 | 0 | α-Na6[B9O15]2(3D) 2(D + P) β-Na6[B9O15]2(2D) 2(P + T + t) γ-form – 0 | 0 | 0 | Cs[B3O5](3D) (T) | 0 | |||
| 5/19 | - | - | K5[B19O31]3D | - | - | - | |||
| 1/4 | 0 | α-(Na,Ag)4[B8O13]2(3D) 2(T + P) β modification – 0 | 0 | 0 | 0 | 0 | |||
| 1/5 | α, β and γ phases – 0 | α-K2[B5O8]2(3D) 2(P) β-K2[B5O8]2(3D) 2(P), γ phase - 0 | α phase – 0 β-Rb2[B5O8]2(3D) 2(P) | α, β, γ phases - 0 | 0 | ||||
| 1/9 | 0 | α, β, and γ phases – 0 | 0 | 0 | Cs2[B9O14]2(3D) 2(B + T) β phase | - | |||
| II. Borates with Cations of Transition Metals | |||||||||
| N | Cations | ||||||||
| Zn | Cd | Mn | Fe | Co | Ni | Cu | Pb | ||
| Orthoborates (with isolated BO3 triangles) | 3 | - | - | Wiserite Mn3[BO3]F3 | - | - | - | - | - |
| 2 | - | - | Mn2[BO3]F | - | - | - | - | 0 (α and β forms) | |
| 3/2 | α-Zn3[BO3]2 | 0 (α and β forms) | Jimboite Mn3[BO3]2 | - | 0 | Ni3[BO3]2 | 0 | - | |
| 5/4 | 0 (α and β forms) | - | - | - | - | - | - | - | |
| Pyroborates (with isol.2) | 1 | - | Cd2[B2O5] | Mn2[B2O5] | Fe2[B2O5] | Co2[B2O5] | 0 | - | 0 (α and β forms) |
| Metaborates (skeletal) | 1/2 | 0 (α and β forms) Zn4O[B6O12] * (t) | - | 0 | 0 | 0 | 0 | Cu3[(B3O6)2] (t) | 0 |
| Polyborates (skeletal) | 3/7 | cub., rhomb. and trig. F, Cl, Br, I and NO3 “boracites” | Cl, Br, I and NO3 “boracites” | cub., rhomb. and trig. F, Cl, Br, I and NO3 “boracites” | cub. and trig. F, Cl, Br, I and NO3 “boracites” | cub., rhomb. and trig. F, Cl, Br, I and NO3 “boracites” | cub. and rhomb. Cl, Br, I and NO3 “boracites” | cub. and rhomb. Cl, Br, I and NO3 “boracites” | - |
| 1/3 | - | 0 | - | - | - | - | - | - | |
| 1/4 | Zn2[B4O7]2 2D | Cd2[B4O7]2 2D | Mn2[B4O7]2 2D | - | - | - | - | Pb[B4O7] (t) | |
| 1/6 | 0 | - | 0 | - | - | - | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonyuk, N.I.; Maltsev, V.V.; Volkova, E.A. Crystal Chemistry of High-Temperature Borates. Molecules 2020, 25, 2450. https://doi.org/10.3390/molecules25102450
Leonyuk NI, Maltsev VV, Volkova EA. Crystal Chemistry of High-Temperature Borates. Molecules. 2020; 25(10):2450. https://doi.org/10.3390/molecules25102450
Chicago/Turabian StyleLeonyuk, Nikolay I., Victor V. Maltsev, and Elena A. Volkova. 2020. "Crystal Chemistry of High-Temperature Borates" Molecules 25, no. 10: 2450. https://doi.org/10.3390/molecules25102450