3.1.4. Final Compounds
(S)-N-(1-Oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)isonicotinamide, 10, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.10 g, 0.30 mmol) and isonicotinic acid were transformed to afford the title compound as a pale-yellow solid (76 mg, 72%); Rf 0.15 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3261 (N–H), 3095, 2973 (C–H), 1638 (C=O), 1617, 1536, 1327 (C–F), 1158 (C–N), 1102, 1065 (C–F) cm−1; δH (400 MHz, DMSO-d6) 10.05 (1H, s, CONHNH), 8.98 (1H, d, J 7, PyCONH), 8.73 (2H, d, J 6, Py-H), 8.39 (1H, bs, CONHNH), 7.82 (2H, d, J 6, Py-H), 7.44 (2H, d, J 8, Ar–H), 6.83 (2H, d, J 8, Ar–H), 4.50 (1H, m, NHCH(CH3)CO), 1.44 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 172.6 (CONHNH), 165.4 (PyCONH), 152.9 (ipso-Ar–C), 150.6 (Py-C), 141.4 (ipso-Py-C), 126.6 (Ar–C), 124.4 (1C, q, 1JC–F 270, CF3), 122.0 (Py-C), 118.5 (1C, q, 2JC–F 31, ipso-Ar-CCF3), 111.9 (Ar–C), 48.7 (NHCH(CH3)CO), 18.0 (NHCH(CH3)CO); δF (282 MHz, DMSO-d6) -59.28 (3F, s, CF3); m/z (ES+) 353 (MH+); HRMS (ES+) found MH+, 353.1237 (C16H16F3N4O2 requires 353.1225).
(S)-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)isonicotinamide, 11, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (0.14 g, 0.41 mmol) and isonicotinic acid were transformed to afford the title compound as a white solid (108 mg, 64%); m.p. 179–181 °C; νmax (ATR) 3264, 1665, 1639, 1615, 1531, 1480, 1410, 1323, 1236, 1158, 1105, 1065, 1010, 878, 836, 757 cm−1; δH (300 MHz, DMSO-d6) 10.12 (1H, s, CONHNH), 8.98 (1H, d, J 7, PyCONH), 8.74 (2H, d, J 6, Py-H), 8.43 (1H, bs, CONHNH), 7.84 (2H, d, J 6, Py-H), 7.45 (2H, d, J 8, Ar–H), 6.82 (2H, d, J 8, Ar–H), 4.61 (1H, m, NHCH(CH2CH2SCH3)), 2.67–2.53 (2H, m, NHCH(CH2CH2SCH3)), 2.15–2.09 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.7 (CONHNH), 165.3 (PyCONH), 150.6 (Py-C), 126.6 (Ar–C), 122.1 (Py-C), 111.9 (Ar–C), 52.3 (NHCH(CH2CH2SCH3)), 31.3 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); δF (282 MHz, DMSO-d6) -59.28 (3F, s, CF3); m/z (ES+) 413 (MH+); HRMS (ES+) found MH+, 413.1261 (C18H20N4O2S requires 413.1259).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)isonicotinamide, 12, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.10 g, 0.32 mmol) and isonicotinic acid were transformed to afford the title compound as a white solid (60 mg, 59%); Rf 0.13 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3302, 3254 (N–H), 3035 (Ar–CH), 2983, 2930 (C–H), 1668, 1638 (C=O) 1599, 1537, 1511 (C=C) 1106 cm−1; δH (400 MHz, DMSO-d6) 9.97 (1H, s, CONHNH), 8.99 (1H, d, J 7, Py-CONH), 8.75 (2H, d, J 6, Py-H), 8.08 (1H, s, CONHNH), 7.84 (2H, d, J 6, Py-H), 7.14 (1H, t, J 8, Ar–H), 6.75–6.66 (3H, m, Ar–H), 4.53 (1H, m, NHCH(CH3)CO), 1.44 (3H, d, J 7, NHCH(CH3)CO); δC (100MHz, DMSO-d6) 172.6 (CONHNH), 165.4 (Py-CO), 151.3 (ipso-Ar–C), 150.6 (Py-C), 141.3 (ipso-Py-C), 133.9 (ipso-Ar–C), 130.5 (Ar–C), 122.0 (Py-C), 118.3 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 48.7 (NHCH(CH3)CO), 17.9 (NHCH(CH3)CO); m/z (ES+) 319 ([37Cl]MH+), 321 ([35Cl]MH+); HRMS (ES+) found [35Cl]MH+, 319.0962 (C15H16N4O235Cl requires 319.0962).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)isonicotinamide, 13, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (0.35 g, 1.13 mmol) and isonicotinic acid were transformed to afford the title compound as a brown solid (113 mg, 26%); m.p. 78–81 °C; νmax (ATR) 3259, 2919, 1659, 1622, 1597, 0521, 1475, 1407, 1385, 1310, 1229, 1151, 1096, 1065, 992, 834 cm−1; δH (300 MHz, DMSO-d6) 10.03 (1H, s, CONHNH), 8.98 (1H, d, J 7, PyCONH), 8.75 (2H, d, J 6, Py-H), 8.09 (1H, bs, CONHNH), 7.84 (2H, d, J 6, Py-H), 7.15 (1H, t, J 8, Ar–H), 6.78–6.62 (3H, m, Ar–H), 4.60 (1H, m, NHCH(CH2CH2SCH3)), 2.67–2.53 (2H, m, NHCH(CH2CH2SCH3)), 2.09–1.98 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.6 (CONHNH), 165.8 (PyCONH), 151.3 (ipso-Ar–C), 150.6 (Py-C), 141.4 (ipso-Ar–C), 134.0 (ipso-Ar–C), 131.3 (ipso-Ar–C), 130.8 (Ar–C), 122.1 (Py-C), 118.3 (Ar–C), 111.9 (Ar–C), 52.3 (NHCH(CH2CH2SCH3)), 31.2 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); m/z (ES+) 379 ([35Cl]MH+), 381 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 379.0994 (C17H20N4O2S35Cl requires 379.0995).
(S)-N-(1-Oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)pyrazine-2-carboxamide, 14, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.10 g, 0.30 mmol) and pyrazinoic acid were transformed following flash chromatography (n-hexane/EtOAc [4:1] to afford the title compound as a white solid (54 mg, 52%); Rf 0.19 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax 3255 (N–H), 3098, 2981 (C–H), 1638 (C=O), 1617, 1534, 1326 (C–F), 1156 (C–N), 1101, 1065 (C–F) cm−1; δH (400 MHz, DMSO-d6) 10.10 (1H, bs, CONHNH), 9.22 (1H, s, Pyz-H), 8.91 (1H, m, Pyz-H), 8.87 (1H, d, J 8, PyzCONH), 8.77 (1H, d, J 2, Pyz-H), 8.43 (1H, bs, CONHNH), 7.48 (2H, d, J 8, Ar–H), 6.84 (2H, d, J 8, Ar–H), 4.60 (1H, m, NHCH(CH3)CO), 1.50 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 172.2 (CONHNH), 163.1 (PyzCO), 152.8 (ipso-Ar–C), 148.2 (Pyz-C), 144.9 (ipso-Pyz-C), 143.99 (Pyz-C), 143.85 (Pyz-C), 126.6 (Ar–C), 123.3 (1C, q, 1JC–F 269, CF3), 118.5 (1C, q, 2JC–F 32, ipso-Ar-CCF3), 111.9 (Ar–C), 47.9 (NHCH(CH3)CO), 18.7 (NHCH(CH3)CO); δF (282 MHz, DMSO-d6) -59.31 (3F, s, CF3); m/z (ES+) 354 (MH+); HRMS (ES+) found MH+, 354.1188 (C15H15F3N5O2 requires 354.1178).
(S)-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)pyrazine-2-carboxamide, 15, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (78 mg, 0.23 mmol) and pyrazinoic acid were transformed to afford the title compound as a yellow semisolid (80 mg, 86%); νmax (ATR) 3264, 1661, 1616, 1519, 1404, 1322, 1157, 1105, 1064, 1019, 838, 832, 771 cm−1; δH (300 MHz, CDCl3) 9.36 (1H, s, Pyz-H), 9.06 (1H, d, J 8, CONHNH), 8.78 (1H, d, J 2, Pyz-H), 8.53 (1H, bs, Pyz-H), 8.43 (1H, d, J 8, CONH), 7.40 (1H, d, J 8, Ar–H), 6.82 (1H, d, J 8, Ar–H), 4.99 (1H, m, NHCH(CH2CH2SCH3)), 2.63 (2H, m, NHCH(CH2CH2SCH3)), 2.35–2.10 (5H, m, NHCH(CH2CH2SCH3); δC (100 MHz, CDCl3) 171.2 (CONHNH), 163.7 (Pyz-CONH), 150.4 (ipso-Ar–C), 147.9 (Pyz-C), 145.0 (ipso-Pyz-C), 144.3 (Pyz-C), 143.4 (ipso-Ar–C), 142.8 (Pyz-C), 126.6 (Ar–C), 122.6 (1C, q, 2JC–F 31, ipso-Ar-CCF3), 112.5 (Ar–C), 50.7 (NHCH(CH2CH2SCH3)), 30.94 (NHCH(CH2CH2SCH3)), 30.15 (NHCH(CH2CH2SCH3)), 15.4 (NHCH(CH2CH2SCH3)); m/z (ES+) 414 (MH+); HRMS (ES+) found MH+, 414.1207 (C17H19F3N5O2S requires 414.1212).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)pyrazine-2-carboxamide, 16, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.10 g, 0.32 mmol) and pyrazinoic acid were transformed to afford the title compound as a white solid (63 mg, 62%) as a mixture of rotamers [6:1]; Rf 0.21 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3383, 3293 (N–H), 3029 (Ar-CH), 2922, 2865 (C–H), 1681, 1659 (C=O) 1596, 1536, 1092, 1018, 886 cm−1; NMR data given for major rotamer δH (400 MHz, CDCl3) 9.35 (1H, s, Pyz-H), 8.89 (1H, s, CONHNH), 8.76 (1H, d, J 2, Pyz-H), 8.53 (1H, m, Pyz-H), 8.34 (1H, d, J 8, Pyz-CONH), 7.06 (1H, dd, J 8, Ar–H), 6.79 (1H, d, J 8, Ar–H), 6.76 (1H, s, Ar–H), 6.65 (1H, d, J 8, Ar–H), 4.85 (1H, m, NHCH(CH3)CO), 1.57 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, CDCl3) 172.1 (CONHNH), 163.5 (Pyz-CONH), 149.0 (ipso-Ar–C), 147.8 (Pyz-C), 144.3 (Pyz-C), 143.6 (ipso-Pyz-C), 142.8 (Pyz-C), 135.0 (ipso-Ar–C), 130.2 (Ar–C), 121.1 (Ar–C), 113.4 (Ar–C), 111.7 (Ar–C), 47.58 (NHCH(CH3)CO), 17.7 (NHCH(CH3)CO); m/z (ES+) 320 ([35Cl]MH+), 322 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 320.0914 (C14H15N5O235Cl requires 320.0914).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)pyrazine-2-carboxamide, 17, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (70 mg, 0.23 mmol) and pyrazinoic acid were transformed to afford the title compound as a white solid (61 mg, 71%); m.p. 99–102 °C; νmax (ATR) 3333, 3290, 2921, 1708, 1678, 1652, 1596, 1585, 1523, 1482, 1438, 1425, 1404, 1375, 1318, 1299, 1272, 1234, 1221, 1188, 1163, 1114, 1091, 1071, 1052, 1023, 991, 958, 946, 914, 864, 809 cm−1; δH (400 MHz, DMSO-d6) 10.03 (1H, bs, CONHNH), 9.22 (1H, s, Pyz-H), 9.00 (1H, d, J 8, CONH), (1H, s, CONHNH), 8.91 (1H, d, J 2, Pyz-H), 8.78 (1H, bs, Pyz-H), 8.10 (1H, bs, CONHNH), 7.16 (1H, dd, J 8, Ar–H), 6.74–6.62 (3H, m, Ar–H), 4.70 (1H, m, NHCH(CH2CH2SCH3)), 2.59–2.50 (2H, m, NHCH(CH2CH2SCH3)), 2.18–2.03 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.2 (CONHNH), 163.7 (Pyz-CONH), 151.3 (ipso-Ar–C), 148.1 (Pyz-C), 145.0 (ipso-Pyz-C), 144.1 (Pyz-C), 143.8 (Pyz-C), 134.0 (ipso-Ar–C), 130.8 (Ar–C), 118.4 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 51.7 (NHCH(CH2CH2SCH3)), 31.6 (NHCH(CH2CH2SCH3)), 30.2 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); m/z (ES+) 380 ([35Cl]MH+), 382 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 380.0948 (C16H1935ClN5O2S requires 380.0948).
(S)-N-(1-Oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)-2-naphthamide, 18, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.10 g, 0.29 mmol) and 2-napthoic acid were transformed to afford the title compound as a white solid (65 mg, 56%); Rf 0.24 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3329, 3264 (N–H), 3051, 2995, 2854, 1674, 1618 (C=O), 1525, 1325 (C–F), 1160 (C–N), 1095, 1065 (C–F), 834 (C–C) cm−1; δH (400 MHz, DMSO) 10.04 (1H, bs, CONHNH), 8.82 (1H, d, J 2, Np-CONH), 8.55 (1H, s, Np–H), 8.42 (1H, d, J 2, CONHNH), 8.08–7.97 (4H, m, Np–H), 7.66–7.58 (2H, m, Np–H), 7.47 (2H, d, J 8, Ar–H), 6.87 (2H, d, J 8, Ar–H), 4.60 (1H, m, NHCH(CH3)), 1.49 (3H, d, J 7, NHCH(CH3)); δC (176 MHz, DMSO-d6) 173.1 (CONHNH), 166.9 (NpCONH), 153.0 (ipso-Ar–C), 134.7 (ipso-Ar–C), 132.5 (ipso-Ar–C), 131.7 (ipso-Ar–C), 129.3 (Np–C), 128.4 (Np–C), 128.4 (Np–C), 128.2 (Ar–C), 128.1 (Np–C), 127.2 (Np–C), 126.6 (Np–C), 125.0 (Np–C), 123.7 (1C, q, 1JC–F 269, CF3), 118.6 (1C, q, 2JC–F 31, ipso-Ar-CCF3), 112.0 (Ar–C), 48.7 (NHCH(CH3)), 18.2 (NHCH(CH3)); δF (282 MHz, DMSO-d6) -59.3 (3F, s, CF3); m/z (ES+) 402 (MH+); HRMS (ES+) found MH+, 402.1427 (C21H19F3N3O2 requires 402.1429).
(S)-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)-2-naphthamide, 19, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (65 mg, 0.19 mmol) and 2-naphthoic acid were transformed to afford the title compound as a white solid (53 mg, 61%); m.p. 163–166°C; νmax (ATR) 3264, 1666, 1635, 1615, 1520, 1503, 1432, 1325, 1232, 1159, 1103, 1065, 1011, 955, 897, 865, 838 cm−1; δH (300 MHz, DMSO-d6) 10.12 (1H, bs, CONHNH), 8.83 (1H, d, J 7, Np-CONH), 8.56 (1H, s, Np–H), 8.44 (1H, bs, CONHNH), 8.10–7.94 (4H, m, Np–H), 7.65–7.55 (2H, m, Np–H), 7.60 (2H, d, J 8, Ar–H), 6.87 (1H, d, J 8, Ar–H), 4.69 (1H, m, NHCH(CH2CH2SCH3)), 2.74–2.55 (2H, m, NHCH(CH2CH2SCH3)), 2.19–2.06 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 172.1 (CONHNH), 167.4 (CONH), 152.9 (ipso-Ar–C), 134.7 (ipso-Ar–C), 134.0 (ipso-Ar–C), 132.5 (ipso-Ar–C), 131.8 (ipso-Ar–C), 129.3 (Np–C), 128.4 (Np–C), 128.2 (Np–C), 128.1 (Np–C), 127.2 (Np–C), 126.6 (Ar–C), 125.0 (Np–C), 118.8 (1C, q, 2JC–F 32, ipso-Ar-CCF3), 111.9 (Ar–C), 52.3 (NHCH(CH2CH2SCH3)), 31.5 (NHCH(CH2CH2SCH3)), 30.5 (NHCH(CH2CH2SCH3)), 15.2 (NHCH(CH2CH2SCH3)); δF (282 MHz, DMSO-d6) 59.26 (3F, s, CF3); m/z (ES+) 462 (MH+), 484 (MNa+); HRMS (ES+) found MH+, 462.1465 (C23H23F3N3O2S requires 462.1463).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)-2-naphthamide, 20, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.10 g, 0.32 mmol) and 2-napthoic acid were transformed to afford the title compound as a white solid (86 mg, 73%); Rf 0.32 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3331, 3236 (NH), 3062 (Ar-CH), 2983, 2926 (CH), 1634, 1626 (C=O),1599, 1536, 1536 (C=C), 1334 (C–N) cm−1; δH (400 MHz, DMSO-d6) 9.96 (1H, bs, CONHNH), 8.82 (1H, d, J 7, Np-CONH), 8.56 (1H, s, Np–H), 8.07–7.95 (4H, m, Np–H), 7.67–7.57 (2H, m, Np–H), 7.14 (1H, dd, J 8, Ar–H), 6.77 (1H, t, J 2, Ar–H), 6.73–6.69 (2H, m, Ar–H), 4.58 (1H, m, NHCH(CH3)CO), 1.47 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 173.0 (CHCONHNH), 166.9 (Np-CONH), 151.4 (Ar–C), 134.7 (Np–C), 134.0 (ipso-Ar–C), 132.5 (Np–C), 131.8 (Np–C), 130.8 (Ar–C), 129.3 (Np–C), 128.3 (Np–C), 128.2 (Np–C), 128.1 (Np–C), 127.2 (Np–C), 125.0 (Np–C), 118.2 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 48.7 (NHCH(CH3)CO), 18.1 (NHCH(CH3)CO); m/z (ES+) 368 ([35Cl]MH+), 370 ([37Cl]MH+); HRMS (ES+) found ([35Cl]MH+, 368.1170 (C20H19N3O235Cl requires 368.1166).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)-2-naphthamide, 21, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (70 mg, 0.23 mmol) and 2-naphthoic acid were transformed to afford the title compound as a white solid (18 mg, 19%); m.p. 205–208 °C; νmax (ATR) 3224, 1636, 1625, 1597, 1527, 1494, 1452, 1440, 1335, 1303, 1268, 1231, 1201, 1076, 989, 955, 892, 863, 839 cm−1; δH (400 MHz, DMSO-d6) 10.04 (1H, bs, CONHNH), 8.85 (1H, d, J 7, Np-CONH), 8.56 (1H, s, Np–H), 8.11 (1H, bs, CONHNH), 8.10–7.97 (4H, m, Np–H), 7.68–7.54 (2H, m, Np–H), 7.15 (1H, dd, J 8, Ar–H), 6.77 (1H, t, J 2, Ar–H), 6.73–6.65 (2H, m, Ar–H), 4.67 (1H, m, NHCH(CH2CH2SCH3)), 2.68–2.56 (2H, m, NHCH(CH2CH2SCH3)), 2.12–2.06 (5H, m, NHCH(CH2CH2SCH3); δC (176 MHz, DMSO-d6) 172.0 (CHCONHNH), 167.4 (Np-CONH), 151.4 (Ar–C), 134.7 (Np–C), 134.0 (ipso-Ar–C), 132.5 (Np–C), 131.8 (Np–C), 130.8 (Ar–C), 129.3 (Np–C), 128.3 (Np–C), 128.2 (Np–C), 128.1 (Np–C), 127.7 (Np–C), 125.0 (Np–C), 118.3 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 52.3 (NHCH(CH2CH2SCH3)), 31.4 (NHCH(CH2CH2SCH3)), 30.5 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); m/z (ES+) 428 ([35Cl]MH+), 430 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 428.1201 (C22H2335ClN3O2S requires 428.1200).
(S)-N-(1-Oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)-1-naphthamide, 22, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.10 g, 0.29 mmol) and 1-napthoic acid were transformed to afford the title compound as a white solid (54 mg, 46%); Rf 0.24 (DCM/EtOH/ sat. aq. NH3 [200:8:1]; νmax (ATR) 3315, 3242 (N–H), 3074 (C–H), 2999, 2865 (C–H), 1667, 1633 (C=O), 1617, 1536, 1324 (C–F), 1159 (C–N), 1095, 1065 (C–F), 828, 787, 776 (C–C) cm−1; δH (400 MHz, DMSO-d6) 10.10 (1H, bs, CONHNH), 8.83 (1H, d, J 7, NpCONH), 8.47 (1H, bs, CONHNH), 8.28 (1H, d, J 8, Np–H), 8.03 (1H, d, J 8, Np–H), 7.98 (1H, d, J 8, Np–H) 7.68 (1H, d, J 7, Np–H), 7.59–7.50 (3H, m, Np–H), 7.47 (2H, d, J 8, Ar–H), 6.91 (2H, d, J 8, Ar–H), 4.60 (1H, m, NHCH(CH3)CO), 1.44 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 173.1 (CONHNH), 167.3 (NpCONH), 153.0 (ipso-NHNHAr–C), 134.1 (Np–C), 134.0 (Np–C), 133.9 (Np–C), 130.2 (Np–C), 128.6 (Np–C), 126.9 (Np–C), 126.7 (Np–C), 126.6 (Ar–C), 126.1 (Np–C), 125.9 (Np–C), 125.3 (Np–C), 118.4 (1C, q, 2JC–F 32, ipso-Ar-CCF3), 112.0 (Ar–C), 48.5 (NHCH(CH3)CO), 18.0 (NHCH(CH3)CO); δF (282 MHz, DMSO-d6) −59.25 (3F, s, CF3); m/z (ES+) 402 (MH+); HRMS (ES+) found MH+, 402.1433 (C21H19F3N3O2 requires 402.1429).
(S)-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)-1-naphthamide, 23, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (65 mg, 0.19 mmol) and 1-naphthoic acid were transformed to afford the title compound as a white solid (53 mg, 61%); m.p. 189–192 °C; νmax (ATR) 3239, 1671, 1635, 1616, 1592, 1521, 1439, 1414, 1378, 1325, 1256, 1231, 1182, 1104, 1065, 1011, 966, 939, 886, 834 cm−1; δH (300 MHz, DMSO-d6) 10.18 (1H, bs, CONHNH), 8.86 (1H, d, J 7, NpCONH), 8.50 (1H, bs, CONHNH), 8.27 (1H, d, J 8, Np–H), 8.08–7.94 (2H, m, Np–H), 7.66–7.52 (3H, m, Np–H), 7.47 (2H, d, J 8, Ar–H), 6.92 (2H, d, J 8, Ar–H), 4.70 (1H, m, NHCH(CH2CH2SCH3)), 2.74–2.55 (2H, m, NHCH(CH2CH2SCH3)), 2.15–2.01 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 172.1 (CONHNH), 167.3 (NpCONH), 153.0 (ipso-Ar–C), 134.7 (ipso-Ar–C), 133.6 (ipso-Ar–C), 130.4 (ipso-Ar–C), 130.3 (Np–C), 128.6 (Np–C), 127.0 (Np–C), 126.7 (Ar–C), 126.5 (ipso-Ar–C), 126.1 (Np–C), 126.0 (Np–C), 125.4 (Np–C), 125.4 (Np–C), 118.3 (1C, q, 2JC–F 31, ipso-Ar-CCF3), 112.0 (Ar–C), 52.1 (NHCH(CH2CH2SCH3)), 31.4 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); δF (282 MHz, DMSO-d6) 59.27 (3F, s, CF3); m/z (ES+) 462 (MH+), 484 (MNa+); HRMS (ES+) found MH+, 462.1468 (C23H23F3N3O2S requires 462.1463).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)-1-naphthamide, 24, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.10 g, 0.32 mmol) and 1-napthoic acid were transformed to afford the title compound as a white solid (55 mg, 45%); νmax (ATR) 3404, 3237 (NH), 3052 (Ar-CH), 2995, 2946 (CH), 1634 (C=O), 1595 (C=C), 1324 (C–N) cm−1; δH (400 MHz, DMSO-d6) 10.00 (1H, bs, CONHNH), 8.83 (1H, d, J 7, Np-CONH), 8.32 (1H, dd, J 8, 2, Np–H), 8.15 (1H, s, CONHNH), 8.03 (1H, d, J 8, Np–H), 7.98 (1H, dd, J 8, 2, Np–H), 7.68 (1H, d, J 8, Np–H), 7.60–7.50 (2H, m, Np–H), 7.16 (1H, dd, J 8, Ar–H), 6.83 (1H, bs, Ar–H), 6.76–6.71 (2H, m, Ar–H), 4.59 (1H, m, NHCH(CH3)CO), 1.42 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 173.1 (CONHNH), 169.2 (Np-CONH), 151.4 (Ar–C), 134.6 (Np–C), 134.0 (Ar–C), 133.5 (Np–C), 130.8 (Ar–C), 130.3 (Np–C), 128.5 (Np–C), 127.0 (Np–C), 126.6 (Np–C), 126.2 (Np–C), 126.0 (Np–C), 125.3 (Np–C), 118.3 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 48.4 (NHCH(CH3)CO), 18.0 (NHCH(CH3)CO); m/z (ES+) 368 ([35Cl]MH+), 370 ([37Cl]MH+); HRMS (ES+) found ([35Cl]MH+, 368.1170 (C20H19N3O235Cl requires 368.1166).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)-1-naphthamide, 25, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (70 mg, 0.23 mmol) and 1-naphthoic acid were transformed to afford the title compound as a pale yellow solid (54 mg, 56%); m.p. 183–186 °C; νmax (ATR) 3239, 1667, 1635, 1594, 1524, 1471, 1435, 1313, 1255, 1216, 1074, 992, 885, 851, 765 cm−1; δH (300 MHz, DMSO-d6) 10.07 (1H, bs, CONHNH), 8.87 (1H, d, J 7, NpCONH), 8.27 (1H, d, J 8, Np–H), 8.15 (1H, bs, CONHNH), 8.05–7.93 (2H, m, Np–H), 7.69 (1H, m, Np–H), 7.63–7.50 (3H, m, Np–H), 7.17 (1H, t, J 6, Ar–H), 6.82 (1H, s, Ar–H), 6.76–6.70 (2H, m, Ar–H), 4.68 (1H, m, NHCH(CH2CH2SCH3)), 2.70–2.58 (2H, m, NHCH(CH2CH2SCH3)), 2.15–2.01 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 172.1 (CONHNH), 169.5 (NpCONH), 151.4 (ipso-Ar–C), 134.6 (ipso-Ar–C), 134.0 (ipso-Ar–C), 130.4 (ipso-Ar–C), 130.3 (Np–C), 128.6 (Np–C), 127.1 (Np–C), 126.7 (Ar–C), 126.7 (ipso-Ar–C), 126.1 (Np–C), 125.4 (Np–C), 118.3 (ipso-Ar–C), 112.0 (Ar–C), 111.4 (Ar–C), 52.1 (NHCH(CH2CH2SCH3)), 31.4 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); m/z (ES+) 428 ([35Cl]MH+), 430 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 428.1192 (C22H23N3O2S35Cl requires 428.1200).
(S)-N-(1-Oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)quinoline-3-carboxamide, 26, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.10 g, 0.30 mmol) and 3-quinolinecarboxylic acid were transformed to afford the title compound as a white solid (53 mg, 44%); Rf 0.15 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3259 (N–H), 2998, 2935 (C–H), 1637, 1617 (C=O), 1522, 1450, 1325, 1158, 1103 1065 (C–F) cm−1; δH (400 MHz, DMSO-d6) 10.09 (1H, bs, CONHNH), 9.34 (1H, d, J 2, Quin-H), 9.06 (1H, d, J 7, QuinCONH), 8.93 (1H, s, Quin-H), 8.43 (1H, s, CONHNH), 8.11 (2H, m, Quin-H), 7.89 (1H, dd, J 8, Quin-H), 7.71 (1H, dd, J 8, Quin-H), 7.47 (2H, d, J 8, Ar–H), 6.87 (2H, d, J 8, Ar–H), 4.63 (1H, quin, J 7, NHCH(CH3)CO), 1.50 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 172.8 (CONHNH), 165.6 (Quin-CO), 152.9 (ipso-Ar–C), 149.7 (Quin-C), 148.9 (ipso-Ar–C), 136.4 (Quin-C), 131.7 (Quin-C), 129.6 (ipso-Ar–C), 129.2 (Quin-C), 127.9 (Quin-C), 127.1 (ipso-Ar–C), 126.6 (ipso-Ar–C), 124.2 (1C, q, 1JC–F 272, Ar-CCF3), 118.6 (1C, q, 2JC–F 31, ipso-Ar-CCF3), 111.9 (Ar–C), 48.8 (NHCH(CH3)CO), 18.1 (NHCH(CH3)CO); δF (282 MHz, DMSO-d6) -59.28 (3F, s, CF3); m/z (ES+) 403 (MH+); HRMS (ES+) found MH+, 403.1367 (C20H18F3N4O2 requires 403.1382).
(S)-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)quinoline-3-carboxamide, 27, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (65 mg, 0.19 mmol) and 3-quinolinecarboxylic acid were transformed to afford the title compound as a beige solid (57 mg, 65%); m.p. 138–141 °C; νmax (ATR) 3258, 3001, 1636, 1616, 1538, 1496, 1418, 1328, 1231, 1185, 1158, 1098, 1066, 1009, 959, 917, 902, 865, 832 cm−1; δH (300 MHz, DMSO-d6) 10.15 (1H, bs, CONHNH), 9.34 (1H, d, J 2, Quin-H), 9.06 (1H, d, J 7, QuinCONH), 8.93 (1H, s, Quin-H), 8.45 (1H, s, CONHNH), 8.12 (2H, m, Quin-H), 7.88 (1H, dd, J 8, Quin-H), 7.71 (1H, dd, J 8, Quin-H), 7.47 (2H, d, J 8, Ar–H), 6.85 (2H, d, J 8, Ar–H), 4.70 (1H, m, NHCH(CH2CH2SCH3)), 2.75–2.56 (2H, m, NHCH(CH2CH2SCH3)), 2.20–2.05 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.9 (CONHNH), 166.0 (Quin-CO), 152.9 (ipso-Ar–C), 149.7 (Quin-C), 148.9 (ipso-Ar–C), 136.4 (Quin-C), 131.7 (Quin-C), 129.6 (ipso-Ar–C), 129.3 (Quin-C), 127.9 (Quin-C), 127.1 (ipso-Ar–C), 126.9 (Ar–C), 123.7 (1C, q, 1JC–F 270, Ar-CCF3), 118.6 (1C, q, 2JC–F 31, ipso-Ar-CCF3), 112.0 (Ar–C), 52.4 (NHCH(CH2CH2SCH3)), 31.4 (NHCH(CH2CH2SCH3)), 30.5 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); δF (282 MHz, DMSO-d6) -59.27 (3F, s, CF3); m/z (ES+) 463 (MH+); HRMS (ES+) found MH+, 463.1416 (C22H22F3N4O2S requires 463.1416).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)quinoline-3-carboxamide, 28, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.10 g, 0.32 mmol) and 3-quinolinecarboxylic acid were transformed to afford the title compound as a white solid (37 mg, 30%); Rf 0.29 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3324 (N–H), 2988, 2947 (C–H), 1646, 1613 (C=O), 1513 (C=N), 1151, 1103, 1065 (C–F) cm−1; δH (400 MHz, DMSO-d6) 10.00 (1H, bs, CONHNH), 9.35 (1H, d, J 2, Quin-NCH), 9.08 (1H, d, J 7, Quin-CONH), 8.93 (1H, s, Quin-CH), 8.15–8.07 (3H, m, Quin-CH, CONHNH), 7.89 (1H, t, J 8, Quin-CH), 7.72 (1H, t, J 8, Quin-CH), 7.15 (1H, t, J 8, Ar–H), 6.78 (1H, t, J 2, Ar–H), 6.71 (2H, m, Ar–H), 4.60 (1H, m, NHCH(CH3)), 1.48 (3H, d, J 7, NHCH(CH3)); δC (75 MHz, DMSO-d6) 172.7 (CONHNH), 165.6 (Quin-CONH), 151.4 (ipso-Ar–C), 149.7 (Quin-C), 149.0 (ipso-Quin-C), 136.4 (Quin-C), 134.0 (ipso-Ar–C), 131.7 (Quin-C), 130.8 (Ar–C), 129.6 (Quin-C), 129.3 (Quin-C), 127.9 (Quin-C), 127.1 (ipso-Ar–C), 126.9 (ipso-Ar–C), 118.3 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 48.7 (NHCH(CH3)), 18.1 (NHCH(CH3)); m/z (ES+) 369 ([35Cl]MH+), 371 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 369.1120 (C19H18N4O235Cl requires 369.1118).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)quinoline-3-carboxamide, 29, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (70 mg, 0.23 mmol) and 3-quinolinecarboxylic acid were transformed to afford the title compound as a white solid (68 mg, 71%); m.p. 141–144 °C; νmax (ATR) 3211, 3029, 2913, 1664, 1638, 1595, 1570, 1534, 1492, 1424, 1374, 1307, 1272, 1229, 1203, 1155, 1136, 1112, 1080, 1019, 993, 981, 960, 948, 914, 900, 876, 855 cm−1; δH (400 MHz, DMSO-d6) 10.08 (1H, bs, CONHNH), 9.35 (1H, d, J 2, Quin-NCH), 9.07 (1H, d, J 7, Quin-CONH), 8.93 (1H, s, Quin-CH), 8.15–8.07 (3H, m, Quin-CH, CONHNH), 7.89 (1H, t, J 8, Quin-CH), 7.72 (1H, t, J 8, Quin-CH), 7.15 (1H, t, J 8, Ar–H), 6.77 (1H, t, J 2, Ar–H), 6.70 (2H, m, Ar–H), 4.67 (1H, m, NHCH(CH2CH2SCH3)), 2.71–2.59 (2H, m, NHCH(CH2CH2SCH3)), 2.16–2.06 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.8 (CONHNH), 166.0 (Quin-CONH), 151.4 (ipso-Ar–C), 149.7 (Quin-C), 149.0 (ipso-Quin-C), 136.4 (Quin-C), 134.0 (ipso-Ar–C), 131.7 (Quin-C), 130.8 (Ar–C), 129.6 (Quin-C), 129.3 (Quin-C), 127.9 (Quin-C), 127.1 (ipso-Ar–C), 126.9 (ipso-Ar–C), 118.3 (Ar–C), 111.9 (Ar–C), 111.4 (Ar–C), 52.4 (NHCH(CH2CH2SCH3)), 31.4 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); m/z (ES+) 429 ([35Cl]MH+), 431 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 429.1148 (C21H2235ClN4O2S requires 429.1152).
(S)-3,5-Dinitro-N-(1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)benzamide, 30, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.10 g, 0.30 mmol) and 3,5-dinitrobenzoic acid were transformed to afford the title compound as a white solid (79 mg, 60%); νmax (ATR) 3250 (NH), 3101 (Ar-CH), 2896 (CH), 1641 (C=O), 1617 (Ar C–C), 1535, 1342, 1330, 1160, 1107, 1066 (C–F) cm−1; δH (400 MHz, DMSO-d6) 10.13 (1H, bs, CONHNH), 9.47 (1H, d, J 7, ArCONH), 9.14 (2H, d, J 2, Ar–H), 8.98 (1H, dd, J 2, Ar–H), 8.43 (1H, bs, CONHNH), 7.47 (2H, d, J 8, Ar–H), 6.84 (2H, d, J 8, Ar–H), 4.61 (1H, m, NHCH(CH3)CO), 1.49 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 172.4 (CONHNH), 163.0 (ArCONH), 152.9 (ipso-Ar–C), 148.6 (ipso-Ar–C), 137.1 (ipso-Ar–C), 128.4 (Ar–C), 126.7 (Ar–C), 121.5 (Ar–C), 111.9 (Ar–C), 49.2 (NHCH(CH3)CO), 18.0 (NHCH(CH3)CO); δF (282 MHz, DMSO-d6) -59.34 (3F, s, CF3); m/z (ES+) 442 (MH+); HRMS (ES+) found MH+, 442.0958 (C17H15F3N5O6 requires 442.0974).
(S)-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)-3,5-dinitrobenzamide, 31, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (65 mg, 0.19 mmol) and 3,5-dinitrobenzoic acid were transformed to afford the title compound as a beige solid (36 mg, 38%); m.p. 97–100 °C; νmax (ATR) 3464, 3212, 3125, 3075, 1662, 1633, 1614, 1541, 1440, 1342, 1322, 1251, 1162, 1115, 1063, 1010, 933, 837 cm−1; δH (300 MHz, DMSO-d6) 10.20 (1H, bs, CONHNH), 9.45 (1H, d, J 7, ArCONH), 9.15 (2H, d, J 2, Ar–H), 8.99 (1H, dd, J 2, Ar–H), 8.44 (1H, bs, CONHNH), 7.47 (2H, d, J 8, Ar–H), 6.84 (2H, d, J 8, Ar–H), 4.68 (1H, m, NHCH(CH2CH2SCH3)), 2.74–2.53 (2H, m, NHCH(CH2CH2SCH3)), 2.19–2.04 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.4 (CONHNH), 163.4 (ArCONH), 152.8 (ipso-Ar–C), 148.6 (ipso-Ar–C), 137.0 (ipso-Ar–C), 128.5 (Ar–C), 127.3 (ipso-Ar–C), 126.6 (Ar–C), 121.5 (Ar–C), 118.7 (1C, q, 2JC–F 32, ipso-Ar-CCF3), 111.9 (Ar–C), 52.7 (NHCH(CH2CH2SCH3)), 31.2 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); δF (282 MHz, DMSO-d6) -59.30 (3F, s, CF3); m/z (ES+) 502 (MH+); HRMS (ES+) found MH+, 502.1010 (C19H19F3N5O6S requires 502.1008).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)-3,5-dinitrobenzamide, 32, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.10 g, 0.32 mmol) and 3,5-dinitrobenzoic acid were transformed to afford the title compound as a light brown solid (54 mg, 42%); Rf 0.21 (DCM/EtOH/ sat. aq. NH3 [200:8:1]); νmax (ATR) 3264 (NH), 3094 (Ar-CH), 2983, 2926 (CH), 1651, 1597 (C=O), 1537, 1342 (N–O), 1334 (C=C) cm−1; δH (300 MHz, DMSO-d6) 10.05 (1H, bs, CONHNH), 9.48 (1H, d, J 7, ArCONH), 9.15 (2H, d, J 2, Ar–H), 8.99 (1H, m, Ar–H), 8.10 (1H, bs, CONHNH), 7.14 (1H, t, J 8, Ar–H), 6.76 (1H, t, J 2, Ar–H), 6.73–6.66 (2H, m, Ar–H), 4.57 (1H, m, NHCH(CH3)CO), 1.48 (3H, d, J 7, NHCH(CH3)CO); δC (100 MHz, DMSO-d6) 172.3 (CONHNH), 163.0 (ArCONH), 151.3 (ipso-Ar–C), 148.5 (ipso-Ar–C), 137.1 (ipso-Ar–C), 134.0 (ipso-Ar–C), 130.7 (Ar–C), 128.4 (Ar–C), 118.3 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 49.2 (NHCH(CH3)CO), 17.9 (NHCH(CH3)CO); m/z (ES+) 408 ([35Cl]MH+), 410 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 408.0701 (C16H15N5O635Cl requires 408.0711).
(S)-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)-3,5-dinitrobenzamide, 33, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (100 mg, 0.32 mmol) and 3,5-dinitrobenzoic acid were transformed to afford the title compound as a light brown gum (149 mg, 99%); m.p. 87–89 °C; νmax (ATR) 3259, 3071, 1649, 1640, 1596, 1536, 1476, 1341, 1340, 1320, 1076, 1041, 918, 847 cm−1; δH (300 MHz, DMSO-d6) 10.12 (1H, bs, CONHNH), 9.44 (1H, d, J 7, ArCONH), 9.15 (2H, d, J 2, Ar–H), 8.99 (1H, dd, J 2, Ar–H), 8.10 (1H, bs, CONHNH), 7.15 (1H, t, J 8, Ar–H), 6.78–6.62 (3H, m, Ar–H), 4.68 (1H, m, NHCH(CH2CH2SCH3)), 2.74–2.53 (2H, m, NHCH(CH2CH2SCH3)), 2.19–2.04 (5H, m, NHCH(CH2CH2SCH3); δC (75 MHz, DMSO-d6) 171.4 (CONHNH), 163.4 (ArCONH), 151.2 (ipso-Ar–C), 148.6 (ipso-Ar–C), 137.1 (ipso-Ar–C), 134.0 (ipso-Ar–C), 130.7 (Ar–C), 128.5 (Ar–C), 121.3 (Ar–C), 118.5 (Ar–C), 111.9 (Ar–C), 111.3 (Ar–C), 52.8 (NHCH(CH2CH2SCH3)), 31.2 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 15.1 (NHCH(CH2CH2SCH3)); m/z (ES+) 468 ([35Cl]MH+), 470 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 468.0743 (C18H1935ClN5O6S requires 468.0745).
(S)-6-chloro-2-ethyl-N-(1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)propan-2-yl)imidazo[1,2-a]pyridine-3-carboxamide, 34, following the standard procedure B, tert-butyl (S)-(1-oxo-1-(2-(4-(trifluoromethyl) phenyl)hydrazineyl)propan-2-yl)carbamate (0.1 g, 0.35 mmol) and 6-chloro-2-ethylimidazo[1,2-a]pyridine-3-carboxylic acid were transformed to afford the title compound as a pale yellow solid (69 mg, 43%); m.p. 221–224 °C; νmax. (ATR) 3252, 2967, 2932, 1667, 1609, 1541, 1522, 1493, 1453, 1442, 1391, 1329, 1281, 1251, 1220, 1160, 1097, 1066, 1009, 995, 952, 906 cm−1; δH (400 MHz, DMSO-d6) 10.10 (1H, d, J 2, CONHNH), 9.01 (1H, d, J 2, Ar–H), 8.47 (1H, d, J 2, CONHNH), 8.33 (1H, d, J 7, NHCH(CH3)CO), 7.68 (1H, d, J 9, Ar–H), 7.51–7.45 (3H, m, Ar–H), 6.91 (2H, d, J 8, Ar–H), 4.60 (1H, q, J 7, NHCH(CH3)CO), 3.00 (2H, q, J 7, CH2CH3), 1.46 (3H, d, J 7, NHCH(CH3)CO), 1.26 (3H, t, J 7, CH2CH3); δC (100 MHz, DMSO-d6) 172.8 (CONHNH), 161.1 (CONH), 153.0 (ipso-Ar–C), 151.8 (ipso-Ar–C), 143.7 (ipso-Ar–C), 127.5 (ipso-Ar–C), 126.6 (Ar–C), 125.2 (Ar–C), 120.1 (ipso-Ar–C), 117.7 (Ar–C), 112.0 (Ar–C), 48.3 (NHCH(CH3)CO), 22.2 (CH2CH3), 18.2 (NHCH(CH3)CO), 13.5 (CH2CH3); m/z (ES+) 454 ([35Cl]MH+), 456 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 454.1256 (C20H2035ClF3N5O2 requires 454.1258).
(S)-6-chloro-2-ethyl-N-(4-(methylthio)-1-oxo-1-(2-(4-(trifluoromethyl)phenyl)hydrazineyl)butan-2-yl)imidazo[1,2-a]pyridine-3-carboxamide, 35, following the standard procedure B, tert-Butyl N-[(1S)-3-(methylsulfanyl)-1-{N′-[4-(trifluoromethyl)phenyl]hydrazine carbonyl}propyl]carbamate (65 mg, 0.19 mmol) and 6-chloro-2-ethylimidazo[1,2-a]pyridine-3-carboxylic acid were transformed to afford the title compound as a white solid (53 mg, 55%); m.p. 227–230 °C; νmax (ATR) 3248, 1664, 1612, 1526, 1487, 1417, 1394, 1374, 1328, 1291, 1189, 1158, 1108, 1066, 1013, 1000, 924, 897, 838 cm−1; δH (300 MHz, DMSO-d6) 10.16 (1H, d, J 2, CONHNH), 8.96 (1H, d, J 2, Ar–H), 8.48 (1H, d, J 2, CONHNH), 8.42 (1H, d, J 7, ArCONH), 7.67 (1H, d, J 9, Ar–H), 7.52–7.45 (3H, m, Ar–H), 6.92 (2H, d, J 8, Ar–H), 4.70 (1H, m, NHCH(CH2CH2SCH3)), 3.00 (2H, q, J 7, CH2CH3), 2.75–2.56 (2H, m, NHCH(CH2CH2SCH3)), 2.20–2.05 (5H, m, NHCH(CH2CH2SCH3), 1.28 (3H, t, J 7, CH2CH3); δC (75 MHz, DMSO-d6) 171.9 (CONHNH), 161.6 (CONH), 152.9 (ipso-Ar–C), 151.8 (ipso-Ar–C), 143.7 (ipso-Ar–C), 127.5 (ipso-Ar–C), 126.5 (Ar–C), 125.1 (Ar–C), 120.1 (ipso-Ar–C), 117.7 (Ar–C), 116.3 (ipso-Ar–C), 112.0 (Ar–C), 52.4 (NHCH(CH2CH2SCH3)), 31.2 (NHCH(CH2CH2SCH3)), 30.4 (NHCH(CH2CH2SCH3)), 22.2 (CH2CH3), 15.1 (NHCH(CH2CH2SCH3)), 13.4 (CH2CH3); δF (282 MHz, DMSO-d6) -59.32 (3F, s, CF3); m/z (ES+) 514 ([35Cl]MH+), 516 ([37Cl]MH+); HRMS (ES+) found [35Cl]MH+, 514.1290 (C22H2435ClF3N5O2S requires 514.1291).
(S)-6-chloro-N-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)-2-ethylimidazo[1,2-a]pyridine-3-carboxamide, 36, following the standard procedure B, tert-butyl (S)-(1-(2-(3-chlorophenyl)hydrazineyl)-1-oxopropan-2-yl)carbamate (0.1 g, 0.4 mmol) and 6-chloro-2-ethylimidazo[1,2-a]pyridine-3-carboxylic acid were transformed to afford the title compound as a white solid (28 mg, 17%); m.p. 213–215 °C; νmax. (ATR) 3258, 2978, 1666, 1608, 1605, 1538, 1492, 1455, 1417, 1394, 1370, 1317, 1269, 1248, 1218, 1159, 1139, 1110, 1075, 1044, 993, 950, 906, 850, 802 cm−1; δH (400 MHz, DMSO-d6) 10.00 (1H, d, J 2, CONHNH), 9.07 (1H, d, J 2, Ar–H), 8.31 (1H, d, J 7, NHCH(CH3)CO), 8.13 (1H, d, J 2, CONHNH), 7.68 (1H, d, J 9, Ar–H), 7.48 (1H, dd, J 9, 2, Ar–H), 7.15 (1H, t, J 6, Ar–H), 6.85 (1H, s, Ar–H), 6.74–6.70 (2H, m, Ar–H), 4.54 (1H, q, J 7, NHCH(CH3)CO), 3.00 (2H, q, J 7, CH2CH3), 1.46 (3H, d, J 7, NHCH(CH3)CO), 1.28 (3H, t, J 7, CH2CH3); δC (100 MHz, DMSO-d6) 172.8 (CONHNH), 161.1 (CONH), 151.9 (ipso-Ar–C), 151.3 (ipso-Ar–C), 143.7 (ipso-Ar–C), 134.1 (ipso-Ar–C), 130.7 (Ar–C), 127.6 (Ar–C), 125.3 (Ar–C), 120.1 (ipso-Ar–C), 118.3 (ipso-Ar–C), 117.7 (Ar–C), 116.3 (ipso-Ar–C), 112.0 (Ar–C), 111.4 (Ar–C), 48.3 (NHCH(CH3)CO), 22.2 (CH2CH3), 18.1 (NHCH(CH3)CO), 13.4 (CH2CH3); m/z (ES+) 420 ([35,35Cl]MH+), 422 ([35,37Cl]MH+), 424 ([37,37Cl]MH+); HRMS (ES+) found [35,35Cl]MH+, 420.0992 (C19H2035,35Cl2N5O2 requires 420.0994).
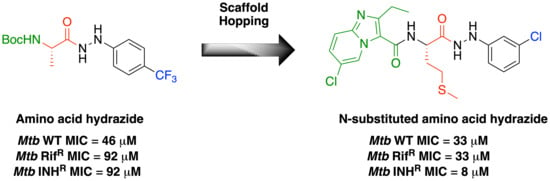
(S)-6-chloro-N-(1-(2-(3-chlorophenyl)hydrazineyl)-4-(methylthio)-1-oxobutan-2-yl)-2-ethylimidazo[1,2-a]pyridine-3-carboxamide, 37, following the standard procedure B, tert-Butyl N-[(1S)-1-[N′-(3-chlorophenyl)hydrazinecarbonyl]-3-(methylsulfanyl)propyl] carbamate (70 mg, 0.23 mmol) and 6-chloro-2-ethylimidazo[1,2-a]pyridine-3-carboxylic acid were transformed to afford the title compound as a white solid (20 mg, 18%); m.p. 188–191 °C; νmax (ATR) 3334, 2916, 1666, 1603, 1519, 1489, 1417, 1396, 1341, 1305, 1288, 1212, 1151, 1120, 1048, 998, 963, 872, 847, 814 cm−1; δH (400 MHz, DMSO-d6) 10.07 (1H, d, J 2, CONHNH), 9.04 (1H, d, J 2, Ar–H), 8.41 (1H, d, J 7, CONH), 8.15 (1H, d, J 2, CONHNH), 7.70 (1H, d, J 9, Ar–H), 7.48 (1H, dd, J 9, 2, Ar–H), 7.16 (1H, t, J 6, Ar–H), 6.86 (1H, s, Ar–H), 6.76–6.68 (2H, m, Ar–H), 4.59 (1H, q, J 7, NHCH(CH2CH2SCH3)), 3.00 (2H, q, J 7, CH2CH3), 2.68–2.56 (2H, m, NHCH(CH2CH2SCH3)), 2.12–2.06 (5H, m, NHCH(CH2CH2SCH3), 1.28 (3H, t, J 7, CH2CH3); δC (176 MHz, DMSO-d6) 172.0 (CONHNH), 161.6 (CONH), 152.0 (ipso-Ar–C), 151.3 (ipso-Ar–C), 143.8 (ipso-Ar–C), 134.1 (ipso-Ar–C), 130.7 (Ar–C), 127.6 (Ar–C), 125.3 (Ar–C), 120.1 (ipso-Ar–C), 118.3 (ipso-Ar–C), 117.7 (Ar–C), 116.4 (ipso-Ar–C), 112.0 (Ar–C), 111.4 (Ar–C), 52.0 (NHCH(CH2CH2SCH3)), 31.2 (NHCH(CH2CH2SCH3)), 31.1 (NHCH(CH2CH2SCH3)), 22.3 (CH2CH3), 15.1 (NHCH(CH2CH2SCH3)), 13.6 (CH2CH3); m/z (ES+) 480 ([35,35Cl]MH+), 482 ([35,37Cl]MH+), 484 ([37,37Cl]MH+); HRMS (ES+) found [35,35Cl]MH+, 480.1026 (C21H2435,35Cl2N5O2S requires 480.1028).