Modification of Chitosan Membranes via Methane Ion Beam
Abstract
:1. Introduction
2. Results and Discussions
2.1. Theory
2.2. XRD
2.3. Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) Spectroscopy Characterization
2.4. Surface Morphology Studies
2.5. Contact Angle Measurements
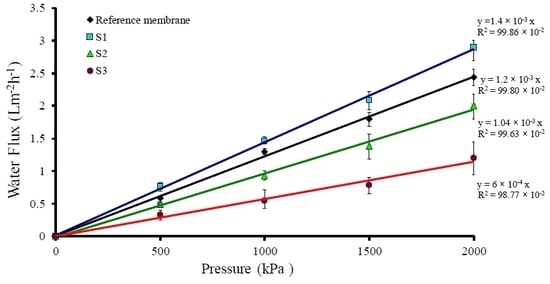
2.6. Water Flux Characterizations
3. Materials and Methods
3.1. Materials
3.2. Ion Beam Bombardment
3.3. Methods and Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baroudi, A.; García-Payo, C.; Khayet, M. Structural, Mechanical, and Transport Properties of Electron Beam-Irradiated Chitosan Membranes at Different Doses. Polyme 2018, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.H.; Du, Y.M.; Yang, J.H. Preparation and anticoagulant activity of carboxybutyrylated hydroxyethyl chitosan sulfates. Carbohydr. Polyme 2003, 51, 431–438. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, B.; Zeng, L.; Zhang, Z.; Liu, Y.; Du, Y.; Xiao, L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. Food Chem. 2004, 84, 107–115. [Google Scholar] [CrossRef]
- Biro, L.P.; Gyulai, J.; Havancsak, K. Scanning probe microscopy investigation of nanometer structures produced by irradiation with 200 MeV ions. Vaccum 1998, 50, 263–272. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Hu, Y.; Kennedy, J.F. Interaction between chitosan and alkyl β-d-glucopyranoside and its effect on their antimicrobial activity. Carbohydr. Polym. 2004, 56, 243–250. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, S.K. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Cabohydr. Polym. 2000, 41, 133–144. [Google Scholar] [CrossRef]
- Goncalves, V.L.; Laranjeira, M.C.M.; Favere, V.T.; Pedrosa, R.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polimeros 2005, 15, 6–12. [Google Scholar] [CrossRef]
- El-Badry, B.A.; Zaki, M.F.; Abdul-Kader, A.M.; Hegazy, T.M.; Morsy, A.A. Ion bombardment of Poly-Allyl-Diglycol-Carbonate (CR-39). Vacuum 2009, 83, 1138–1142. [Google Scholar] [CrossRef]
- Garcia, J.A.; Rodriguez, R.J. Ion implantation techniques for non-electronic applications. Vacuum 2011, 85, 1125–1129. [Google Scholar] [CrossRef]
- Wanichapichart, P.; Taweepreeda, W.; Choomgan, P.; Yu, L.D. Argon and nitrogen beams influencing membrane permeate fluxes and microbial growth. Radiat. Phys. Chem. 2010, 79, 214–218. [Google Scholar] [CrossRef]
- James, J.; Joseph, B.; Shaji, A.; Nancy, P.; Kalarikkal, N.; Thomas, S.; Grohens, Y.; Vignaud, G. Microscopic Analysis of Plasma-Activated Polymeric Materials. In Non-Thermal Plasma Technology for Polymeric Materials; Sabu, T., Miran, M., Uroš, C., Petr, Š., Praveen, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 11; pp. 287–317. [Google Scholar] [CrossRef]
- Prakrajang, K.; Wanichapichart, P.; Anuntalabhochai, S.; Pitakrattananukool, S.; Yu, L.D. Ion beam modification of chitosan and cellulose membranes for simulation of ion bombardment of plant cell envelope. Nucl. Instrum. Meth. Phys. Res. B 2009, 267, 1645–1649. [Google Scholar] [CrossRef]
- Jaleh, B.; Parvin, P.; Wanichapichart, P.; Saffar, A.P.; Reyhani, A. Induced super hydrophilicity due to surface modification of polypropylene membrane treated by O2 plasma. Appl. Surf. Sci. 2010, 257, 1655–1659. [Google Scholar] [CrossRef]
- Jaleh, B.; Parvin, P.; Sheikh, N.; Zamanipour, Z.; Sajad, B. Hydrophilicity and morphological investigation of polycarbonate irradiated by ArF excimer laser. Nucl. Instrum. Meth. Phys. Res. B 2007, 265, 330–333. [Google Scholar] [CrossRef]
- Jaleh, B.; Shayegani Madad, M.; Farshchi Tabrizi, M.; Habibi, S.; Golbedaghi, R.; Keymanesh, M.R. UV-degradation effect on optical and surface properties of polystyrene-TiO2 nanocomposite film. J. Iran. Chem. Soc. 2011, 8, 161–168. [Google Scholar] [CrossRef]
- Jaleh, B.; Parvin, P.; Sheikh, N.; Ziaie, F.; Haghshenas, M.; Bozorg, L. Evaluation of physico-chemical properties of electron beam-irradiated polycarbonate film. Radiat. Phys. Chem. 2007, 76, 1715–1719. [Google Scholar] [CrossRef]
- Sofield, C.J.; Sugden, S.; Ing, J.; Bridwell, L.B.; Wang, Y.Q. Ion beam modification of polymers. Vacuum 1993, 44, 285–290. [Google Scholar] [CrossRef]
- Velardi, L.; Lorusso, A.; Paladini, F.; Siciliano, M.V.; Giulio, M.; Raino, A.; Nassisi, V. Modification of polymer characteristics by laser and ion beam. Radiat. Eff. Defects Solids 2010, 165, 637–642. [Google Scholar] [CrossRef]
- Khan, K.A.; Salmieri, S.; Dussault, D.; URIBE-CALDERON, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef]
- Jaleh, B.; Gavary, N.; Fakhri, P.; Muensit, N.; Taheri, S.M. Characteristics of PVDF membranes irradiated by electron beam. Membranes 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jaleh, B.; Etivand, E.S.; Mohazzab, B.F.; Nasrollahzadeh, M.; Varma, R.S. Improving wettability: Deposition of TiO2 nanoparticles on the O2 plasma activated polypropylene membrane. Int. J. Mol. Sci. 2019, 20, 3309. [Google Scholar] [CrossRef] [Green Version]
- Leveneur, J.; Rajan, A.; McDonald-Wharry, J.; Le Guen, M.J.; Pickering, K.; Kennedy, J. Structural and chemical changes of cellulose fibres under low energy ion implantations. Surf. Coat. Tech. 2018, 355, 191–199. [Google Scholar] [CrossRef]
- Cheng, X.; Kondyurin, A.; Bao, S.; Bilek, M.M.M.; Ye, L. Plasma immersion ion implantation of polyurethane shape memory polymer: Surface properties and protein immobilization. Appl. Surf. Sci. 2017, 416, 686–695. [Google Scholar] [CrossRef]
- Mathakari, N.L.; Bhoraskar, V.N.; Dhole, S.D. MeV energy electron beam induced damage in isotactic polypropylene. Nucl. Instrum. Meth. Phys. Res. B 2008, 266, 3075–3080. [Google Scholar] [CrossRef]
- Endrskt, R.; Skvorckik, V.; Rybka, V.; Hnatowicz, V. Surface modification of polymers indused by ion implantation. Radiat. Eff. Defects Solids 1995, 137, 25–28. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Migdal, W.; Swietoslawski, J.; Jakubaszek, U.; Tarnowski, T. Chemical-radiation degradation of natural oligoamino-polysaccharides for agricultural application. Radiat. Phys. Chem. 2007, 76, 1840–1842. [Google Scholar] [CrossRef]
- Kulshrestha, V.; Awasthi, K.; Acharya, N.K.; Singh, M.; Bhagwat, P.V.; Vijay, Y.K. Structural, optical, thermo-mechanical and transport properties of ion irradiated polymer membranes. Polym. Bull. 2006, 56, 427–435. [Google Scholar] [CrossRef]
- Gryczka, U.; Dondi, D.; Chmielewski, A.G.; Migdal, W.; Buttafava, A.; Faucitano, A. The mechanism of chitosan degradation by gamma and e-beam irradiation. Radiat. Phys. Chem. 2009, 78, 543–548. [Google Scholar] [CrossRef]
- Singh Rathore, B.; Singh Gaur, M.; Singh, F.; Shanker Singh, K. Optical and dielectric properties of 55 MeV carbon beam-irradiated polycarbonate films. Radiat. Eff. Defects Solids. 2012, 167, 131–140. [Google Scholar] [CrossRef]
- Nagata, S.; Konishi, Y.; Tsuchiya, B.; Toh, K.; Yamamoto, S.; Takahiro, K.; Shikama, T. Ion beam effects on electrical characteristics of proton conductive polymer. Nucl. Instr. Meth. Phys. Res. B 2007, 257, 519–522. [Google Scholar] [CrossRef]
- Nasef, M.M.; Saidi, H.; Dahlan, K.Z.M. Effects of APTEOS content and electron beam irradiation on physical and separation properties of hybrid nylon-66 membranes. Nucl. Instr. Meth. Res. B 2007, 265, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Fintzou, A.T.; Badeka, A.V.; Kontominas, M.G.; Ringanakos, K.A. Changes in physicochemical and mechanical properties of γ-irradiated polypropylene syringes as a function of irradiation dose. Radiat. Phys. Chem. 2006, 75, 87–97. [Google Scholar] [CrossRef]
- Murthy, C.S.; Posselt, M.; Frei, T. Three-dimensional modeling of low-dose BF+2 implantation into single-crystalline silicon. J. Vac. Sci. Technol B 1996, 14, 278–282. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Biersack, J.P.; Ziegler, M.D. The Stopping and Range of Ions in Matter; SRIM, Co.: Chester, MD, USA, 2008. [Google Scholar]
- SRIM-2008 Software Package. Available online: http://www.srim.org (accessed on 25 February 2019).
- Popok, V.N. High-fluence ion implantation of polymers: Evolution of structure and composition. In Radiation Effects in Polymeric Materials; Chapter 3; Kumar, V., Chaudhary, B., Sharma, V., Verma, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 69–111. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cartier, N.; Domand, A.; Chanzy, H. Single crystals of chitosan. Int. J. Biol. Macromol. 1990, 12, 289–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.R.; Wang, Y.J.; Ren, L.; Chen, X.F. Surface Modification of Chitosan Membranes by Oxygen Plasma Treatment. Mater. Sci. Forum. 2009, 610, 1259–1262. [Google Scholar] [CrossRef]
- Wanichapichart, P.; Sungkum, R.; Taweepreda, W.; Nisoa, M. Characteristics of chitosan membranes modified by argon plasmas. Surf. Coat. Technol. 2009, 203, 2531–2535. [Google Scholar] [CrossRef]
- Li, X.; Shi, X.; Wang, M.; Du, Y. Xylan chitosan conjugate A potential food preservative. Food. Chem. 2011, 126, 520–525. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.; Sudha, P.N.; Venkatesan, J.; Anil, S. Synthesis, Characterization and applications of nanochitosan/sodium alginate/microcrystalline cellulose film. J. Nanomed. Nanotechnol. 2016, 7, 419–429. [Google Scholar] [CrossRef]
- Choi, S.C.; Han, S.; Choi, W.K.; Jung, H.J.; Koh, S.K. Hydrophilic group formation on hydrocarbon polypropylene and polystyrene by ion-assisted reaction in an O2 environment. Nucl. Instr. Meth. Phys. Res. B 1999, 152, 291–300. [Google Scholar] [CrossRef]
- Dworecki, K.; Drabik, M.; Hasegawa, T.; Wasik, S. Modification of polymer membranes by ion implantation. Nucl. Instr. Meth. Phys. Res. B 2004, 225, 483–488. [Google Scholar] [CrossRef]
- Shang, H.M.; Wang, Y.; Takahashi, K.; Cao, G.Z.; Li, D.; Xia, Y.N. Nanostructured superhydrophobic surfaces. J. Mater. Sci. 2005, 40, 3587–3591. [Google Scholar] [CrossRef]
- Sperelakis, N. Cell Physiology Source Book, 4th ed.; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Wanichapichart, P.; Kaewnoparat, S.; Phud-hai, W.; Buaking, K. Characteristic of Filtration Membranes Produced by Acetobacter xylinum. Songklanakarin J. Sci. Technol. 2003, 24, 855–862. [Google Scholar]
- Zhu, F.; Tajkhorshid, E.; Schulten, K. Pressure-Induced Water Transport in Membrane Channels Studied by Molecular Dynamics. J. Biophysical. 2002, 83, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Asenjo, J.A. Separation Processes in Biotechnology. In Bioprocess Technology Serie; Asenjo, J.A., Ed.; Marcel Dekker: New York, NY, USA, 1990; p. 212. [Google Scholar]
- Wanichapichart, P.; Yu, L. Chitosan membrane filtering characteristics modification by N-ion beams. Surf. Coat. Technol. 2007, 201, 8165–8169. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







| E0 (30 keV) | E0 (55 keV) | E0 (80 keV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ec | EH | dc | dH | Ec | EH | dc | dH | Ec | EH | dc | dc | |
| (keV) | (keV) | (Å) | (keV) | (keV) | (keV) | (keV) | (µm) | |||||
| CH4 | 22.5 | 1.875 | 4999 | 2460 Å | 41.25 | 3.428 | 8935 Å | 4220 Å | 60 | 5 | 1.27 | 5807 Å |
| CH3 | 24 | 2 | 5320 | 2680 Å | 44 | 3.66 | 9499 Å | 4455 Å | 64 | 5.33 | 1.35 | 6124 Å |
| CH2 | 25.7 | 2.14 | 5683 | 2772 Å | 47.14 | 3.93 | 1.01 µm | 4737 Å | 68.57 | 5.71 | 1.44 | 6483 Å |
| CH | 27.7 | 2.3 | 6108 | 2958 Å | 50.77 | 4.23 | 1.09 µm | 5077 Å | 73.85 | 6.15 | 1.54 | 6889 Å |
| C | 30 | 6594 | 55 | 4.23 | 1.17 µm | 80 | 1.66 | |||||
| H2 | 15 | 1.37 µm | 27.5 | 2.12 µm | 40 | 2.77 µm | ||||||
| H | 30 | 2.26 µm | 55 | 3.49 µm | 80 | 4.65 µm | ||||||
| Membrane | Crystallinity (%) |
|---|---|
| Reference membrane | 27.7 |
| S1 | 25.9 |
| S2 | 22.4 |
| S3 | 17.3 |
| Band | Vibrations Peak (cm−1) |
|---|---|
| (O–H stretch) | 3400 |
| (C–H stretch) | 2875 |
| (C=O stretch, amide group) | 1640 |
| (N–H deformation, amino group) | 1585 |
| (C–O stretch, amide group) | 1375 |
| (bridge O stretch) | 1155 |
| (C–O stretch) | 1092 |
| Sample | Lp × 10−13 (m3 N−1 S−1) |
|---|---|
| Reference membrane | 3.33 |
| S1 | 3.89 |
| S2 | 2.89 |
| S3 | 1.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, N.; Jaleh, B.; Golbedaghi, R.; Larijani, M.M.; Wanichapichart, P.; Nasrollahzadeh, M.; Varma, R.S. Modification of Chitosan Membranes via Methane Ion Beam. Molecules 2020, 25, 2292. https://doi.org/10.3390/molecules25102292
Gholami N, Jaleh B, Golbedaghi R, Larijani MM, Wanichapichart P, Nasrollahzadeh M, Varma RS. Modification of Chitosan Membranes via Methane Ion Beam. Molecules. 2020; 25(10):2292. https://doi.org/10.3390/molecules25102292
Chicago/Turabian StyleGholami, Nasim, Babak Jaleh, Reza Golbedaghi, Majid Mojtahedzadeh Larijani, Pikul Wanichapichart, Mahmoud Nasrollahzadeh, and Rajender S. Varma. 2020. "Modification of Chitosan Membranes via Methane Ion Beam" Molecules 25, no. 10: 2292. https://doi.org/10.3390/molecules25102292