1. Introduction
Houttuynia cordata Thunb. is a medicinal plant commonly found in Southeast Asia. In China, the young roots and green leaves of
H. cordata are popular vegetable products, being used in the preparation of beverages by boiling decoction.
Houttuynia cordata contains a wide range of compounds including polysaccharides, fatty acids, polyphenols, flavonoids, and sterols, and has anti-viral, antifungal, detoxifying, and anti-bacterial properties [
1]. Hayashi et al. [
2] reported that
H. cordata extract reduces the infectivity (by >4 log) of several viruses, including the influenza virus and HIV. Even though several biological activities of
H. cordata have been studied [
3], the effects of
H. cordata against human noroviruses (HuNoVs) and its mechanism of action remain unknown.
Human noroviruses, which belong to the Caliciviridae family, are the most common cause of foodborne viral gastroenteritis throughout the world. Human noroviruses can be divided into five major genogroups, genogroup I (GI) through genogroup V (GV); Genogroup II.4 (GII.4) HuNoVs are closely related to most foodborne noroviral outbreaks. Human noroviruses infections mainly occur via person-to-person transmission through fecal–oral route or by consuming contaminated food. Human noroviruses are highly tolerant to environmental changes and have low infectious doses of eight to 10 viral particles [
4]. Most sources of foodborne disease caused by HuNoVs are foods prepared with contaminated hands or cookware. Currently, there is no commercially available antiviral drug or vaccine for the prevention of norovirus infections or outbreaks.
The inactivation of HuNoVs mainly relies on physical treatments including chemical methods (e.g., titanium dioxide, sodium hypochlorite, and hydrogen peroxide), heat treatment, and ultraviolet radiation. However, the use of such treatments is frequently inadequate in the food industry. Therefore, it is of utmost importance to investigate safe anti-noroviral agents that can be consumed. Several compounds isolated from plants (e.g., polysaccharides, polyphenols, and flavonoids) have antimicrobial activity [
5]. A recent review suggested that plant extracts containing polysaccharides and polyphenols, such as
Ganoderma lucidum polysaccharide (GLPS), Pericarpium granati extract (PGE), and Pomegranate extract (PE), might prevent infection from NoV surrogates [
6]. These natural products are safe antiviral agents because they can be eaten, and their nutrition-promoting properties have long been studied [
7,
8].
Even though it has been reported that HuNoVs may be cultured in B cells in vitro, there are several disadvantages to this method for screening natural antiviral compounds [
4]. Anti-noroviral effects are evaluated from the reduction in infectivity of cultivatable HuNoV surrogates such as murine norovirus (MNV-1), bacteriophage MS2, or other in vitro models of HuNoV infectivity detection (in situ capture qRT-PCR) [
9,
10,
11]. Among them, MNV-1, a genogroup V (GV) cultivable norovirus, is currently recognized as the most suitable surrogate for HuNoVs [
10].
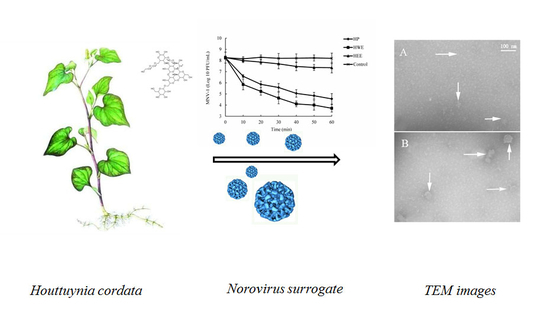
In the study, we identified the anti-norovirus potential of crude water extract (HWE), purified polysaccharide (HP), and ethanol extract (HEE) from H. cordata by plaque assay for MNV-1. Additionally, we investigated the structure, chemical composition, and antiviral mechanism of HP.
3. Discussion
Although most studies have focused on investigating the role of
H. cordata extracts against microorganisms [
21,
22], few studies have evaluated the effectiveness and mechanism of action of
H. cordata extracts against HuNoVs. In this study,
H. cordata extracts prepared in water (HWE and HP) and ethanol (HEE) exhibited various degrees of antiviral activity in a dose- and time-dependent manner. The values of selectivity index (SI) were 1.95 for HEE, 5.74 for HP, and 16.14 for HWE. Samples with high SIs had maximum antiviral activity, minimal cell toxicity, and a wider range of applications [
23]. Among the extracts, HWE, mainly composed of carbohydrates, exhibited the strongest antiviral activity with the highest SI value. According to previous studies, carbohydrates are the main active substances in
H. cordata water extracts and exhibited potent anti-viral activities [
1,
2,
6,
12,
13]. In this study, we analyzed the
H. cordata polysaccharide structures and their anti-noroviral mechanisms.
A polysaccharide (HP), isolated and purified from HWE with a molecular weight < 50 kDa, exhibited stronger anti-noroviral activity with higher SI value than HEE. Structural analysis revealed that HP mainly consists of α-1,4-linked Gal
pA, β-1,4-linked Gal
p, β-1,4-linked Glc
p, and β-1,4-linked Xyl
p residues. The predominant number of Gal
p and Gal
pA residues suggests that HP might be a pectin-like acidic polysaccharide with a 1,4-linked Gal
p core. In addition, 1, 6-linked Gal
p, 1,4,6-linked Gal
p, 1,2,4-linked Rha
p, 1,5-linked Ara
f, and terminal-linked Gal
p and Glc
p in the side chains were detected. It has been proposed that some carbohydrates consisting of 1,4-linked GalpA may prevent the adhesion of some enteric pathogens such as protozoa, bacteria, and virus [
24,
25]. The polysaccharide (HP) is mainly composed of carbohydrates consisting of 1,4-linked GalpA and 1,4-linked Galp. The pectin-like acidic polysaccharide HP with a 1,4-linked Galp core might be the active substance in HWE responsible for the antiviral activity against MNV-1.
The Weibull model was used to evaluate the antiviral behavior of HP during 60 min (
Supplementary Materials Figure S1). Based on the survival curves of MNV-1, the Weibull model showed the best fit to HP treatment with the highest R
2 (>0.90) and lowest RMSE (<0.05) values. The kinetic model for the inactivation of MNV-1 can be analyzed with the
D-value, which represents the time required to reduce the population of pathogens by 90% and can be measured from a time versus log survivors’ curve [
26,
27]. In our study,
D-values decreased with increasing HP concentration. The
D-values for MNV-1 inactivation were different (
p ≤ 0.05) between the low treatments (100 μg/mL) and high treatments (250 and 500 μg/mL).
To further understand the antiviral mechanism of HP, we investigated the effects of HP on high-titer MNV-1 (8.09 log10 PFU/mL) using dose- and time-dependence experiments. Additionally, we observed the morphology of MNV-1 in low titer (4.38 log10 PFU/mL) with TEM under conditions that reduced its infectivity by more than 3 log10 PFU/mL. It has been reported that some polysaccharides inhibit viral infections by blocking the adsorption, entry, and/or cell-to-cell transmission of viruses. In addition, polysaccharides bind to viral envelope glycoproteins and disrupt them with negatively charged carboxylate groups, therefore minimizing/preventing viruses from penetrating target cells [
28,
29]. In our study, the changes in size and morphology of MNV-1 particles following HP treatment demonstrated that the polysaccharide had anti-norovirus activity by denaturing and enlarging virus particles to inhibit virus penetration. The morphological changes in MNV-1 following HP treatment were similar to those obtained in bacteriophage T4 and rotavirus with flavonoid compound CJs (cranberry juices) [
30] and in MNV-1 treated with polyphenolic compound RCS-F1 (raspberry seed extract fraction-1) [
31]. These results suggest that these natural compounds inactivate viruses by a similar mechanism.
In this study, we identified the anti-norovirus potential of HWE, HP, and HEE using a plaque assay for MNV-1. The pectin-like acidic polysaccharide HP, with 1,4-linked Gal
p core, might be the active substance in HWE responsible for the antiviral activity against MNV-1. Currently, the only method for preventing noroviral infections is hand washing. In this study, the antiviral effects of HP and HWE reduced the residual MNV-1 infectivity following 10 min of incubation; therefore, these extracts interact immediately with the virus. The use of these extracts entails no safety concerns because
H. cordata is a permitted food additive in China [
32]. Our findings support the use of HP and HWE as nontoxic agents. Zhu et al. [
33] and Kumar et al. [
34] have shown that
H. cordata exerts strong effects against a large number of enveloped and non-enveloped viruses. Therefore, HP and HWE might be potential antiviral agents in the prevention of viral diseases. However, we used unpurified HWE in this study. It is possible that total phenolics, proteins, and flavonoids in the extract may participate in the inactivation of the MNV-1 in a synergistic manner. It has been reported that a new type of flavonoid (Houttuynoids A–E (1–5)), from the whole plant of
Houttuynia cordata, exhibited potent anti-HSV (herpes simplex viruses) activity [
35]. The determination of the phenolic content in HWE through Folin–Ciocalteu solely corresponds to an estimation of the presence of reducing compounds in this study. Thus, future studies should investigate the characterization of polyphenolic profiles in HWE, and their mechanism of antiviral action.