Application of Response Surface Methodology and Desirability Function in the Optimization of Adsorptive Remediation of Arsenic from Acid Mine Drainage Using Magnetic Nanocomposite: Equilibrium Studies and Application to Real Samples
Abstract
:1. Introduction
2. Results and Discussion
2.1. Brunauer, Emmett, and Teller Surface Area Measurement
2.2. Optimization Strategy
2.2.1. A 23 Full Factorial Design
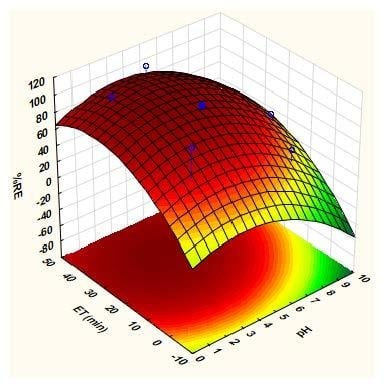
2.2.2. Response Surface Methodology based on Central Composite Design
2.2.3. Optimization of Adsorption Process Using the Desirability Function
2.3. Adsorption Isotherms
2.3.1. Two Parameter Isotherms
2.3.2. Three Parameter Isotherms
2.3.3. Coefficients of Determination
2.4. Regeneration and Recycling of MWCNT-Fe3O4@Zeo Nanocomposite
2.5. Application to Real Samples
3. Materials and Methods
3.1. Instrumentations
3.2. Reagents and Solutions
3.3. Preparation of MWCNT-Fe3O4@Zeo Nanocomposite
3.4. Determination of pH Point of Zero Charge (pHzpc) of MWCNT-Fe3O4@Zeo Nanocomposite
3.5. Multivariate Optimization of the Adsorption Process
3.6. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Wu, M.; Lin, X.; Huang, P.; Huang, Y. Synthesis of magnetic wheat straw for arsenic adsorption. J. Hazard. Mater. 2011, 193, 10–16. [Google Scholar] [CrossRef]
- Adra, A.; Morin, G.; Ona-Nguema, G.; Brest, J. Arsenate and arsenite adsorption onto Al-containing ferrihydrites. Implications for arsenic immobilization after neutralization of acid mine drainage. Appl. Geochem. 2016, 64, 2–9. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Thanawatpoontawee, S.; Imyim, A.; Praphairaksit, N. Iron-loaded zein beads as a biocompatible adsorbent for arsenic (V) removal. J. Ind. Eng. Chem. 2016, 43, 127–132. [Google Scholar] [CrossRef]
- Hao, L.; Wang, N.; Wang, C.; Li, G. Arsenic removal from water and river water by the combined adsorption-UF membrane process. Chemosphere 2018, 202, 768–776. [Google Scholar] [CrossRef]
- Akin, I.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Removal of arsenate [As (V)] and arsenite [As (III)] from water by SWHR and BW-30 reverse osmosis. Desalination 2011, 281, 88–92. [Google Scholar] [CrossRef]
- Hernández-Flores, H.; Pariona, N.; Herrera-Trejo, M.; Hdz-García, H.M.; Mtz-Enriquez, A.I. Concrete/maghemite nanocomposites as novel adsorbents for arsenic removal. J. Mol. Struct. 2018, 1171, 9–16. [Google Scholar] [CrossRef]
- Li, W.; Cao, C.-Y.; Wu, L.-Y.; Ge, M.-F.; Song, W.-G. Superb fluoride and arsenic removal performance of highly ordered mesoporous aluminas. J. Hazard. Mater. 2011, 198, 143–150. [Google Scholar] [CrossRef]
- De Klerk, R.J.; Jia, Y.; Daenzer, R.; Gomez, M.A.; Demopoulos, G.P. Continuous circuit coprecipitation of arsenic (V) with ferric iron by lime neutralization: Process parameter effects on arsenic removal and precipitate quality. Hydrometallurgy 2012, 111, 65–72. [Google Scholar] [CrossRef]
- An, B.; Liang, Q.; Zhao, D. Removal of arsenic (V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Res. 2011, 45, 1961–1972. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Kojima, T.; Basha, C.A.; Srinivasakannan, C. Removal of arsenic from aqueous solution using electrocoagulation. J. Hazard. Mater. 2009, 167, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Gialdini, F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, D.; Pan, R.; Xu, L.; Demopoulos, G.P. A novel two-step coprecipitation process using Fe (III) and Al (III) for the removal and immobilization of arsenate from acidic aqueous solution. Water Res. 2012, 46, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, H.; Ni, J. Arsenate removal by Donnan dialysis: effects of the accompanying components. Sep. Purif. Technol. 2010, 72, 250–255. [Google Scholar] [CrossRef]
- Dalida, M.L.P.; Mariano, A.F.V.; Futalan, C.M.; Kan, C.-C.; Tsai, W.-C.; Wan, M.-W. Adsorptive removal of Cu (II) from aqueous solutions using non-crosslinked and crosslinked chitosan-coated bentonite beads. Desalination 2011, 275, 154–159. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; ALOthman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; Shao, X.; Yuan, S.; Yang, C.; Hu, X. Adsorption of heavy metal ions by hierarchically structured magnetite-carbonaceous spheres. Talanta 2012, 101, 45–52. [Google Scholar] [CrossRef]
- Yen, C.-H.; Lien, H.-L.; Chung, J.-S.; Yeh, H.-D. Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J. Hazard. Mater. 2017, 322, 215–222. [Google Scholar] [CrossRef]
- Hao, L.; Zheng, T.; Jiang, J.; Zhang, G.; Wang, P. Removal of As (III) and As (V) from water using iron doped amino functionalized sawdust: Characterization, adsorptive performance and UF membrane separation. Chem. Eng. J. 2016, 292, 163–173. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Cai, Y.; Zhao, X.; Shi, Y. Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4. Chem. Eng. J. 2010, 158, 599–607. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, S.; Zhang, L.; Wang, C.; Zhang, B. Kinetic, Isotherm, and Thermodynamic Studies for Ag (I) Adsorption Using Carboxymethyl Functionalized Poly (glycidyl methacrylate). Polymers 2018, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Wang, S.; Zhang, L.; Liu, N.; Zhang, B. Selective capture models and mechanisms of Pb (II) from wastewater using tannic-functionalized nickel-iron oxide Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 265–273. [Google Scholar] [CrossRef]
- Gdula, K.; Skwarek, E.; Dąbrowski, A.; Melnyk, I.V. Amine-functionalized silica particles with magnetic core as magnetically removable adsorbents of Ag (I) ions. Adsorpt. Sci. Technol. 2017, 35, 432–438. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Ramutshatsha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Simultaneous removal of Na, Ca, K and Mg from synthetic brine and seawater using Fe2O3-SiO2 mixed oxide nanostructures: Kinetics and isotherms studies. Desalin. Water Treat. 2018, 104, 206–216. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Goudarzi, A.; Rajabi, M. Response surface methodology approach for optimization of simultaneous dye and metal ion ultrasound-assisted adsorption onto Mn doped Fe3O4-NPs loaded on AC: Kinetic and isothermal studies. Dalton Trans. 2015, 44, 14707–14723. [Google Scholar] [CrossRef]
- Sahu, U.K.; Mahapatra, S.S.; Patel, R.K. Application of Box–Behnken Design in response surface methodology for adsorptive removal of arsenic from aqueous solution using CeO2/Fe2O3/graphene nanocomposite. Mater. Chem. Phys. 2018, 207, 233–242. [Google Scholar] [CrossRef]
- Mourabet, M.; El Rhilassi, A.; El Boujaady, H.; Bennani-Ziatni, M.; Taitai, A. Use of response surface methodology for optimization of fluoride adsorption in an aqueous solution by Brushite. Arab. J. Chem. 2017, 10, S3292–S3302. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Iqbal, N.; Bhatti, I.A.; Ahmad, N.; Zahid, M. Response surface methodology application in optimization of cadmium adsorption by shoe waste: A good option of waste mitigation by waste. Ecol. Eng. 2016, 88, 265–275. [Google Scholar] [CrossRef]
- Mashile, G.P.; Mpupa, A.; Dimpe, M.K.; Nomngongo, P.N. Magnetic activated carbon@iron oxide@manganese oxide composite as an adsorbent for preconcentration of microcystin–LR in surface water, tap water, water and wastewater. Environ. Nanotechnol. Monitor. Manag. 2018, 10, 199–205. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.-Z.; Li, W.-G.; Wang, P.; Su, C.-Y. Adsorption of acid and basic dyes by sludge-based activated carbon: Isotherm and kinetic studies. J. Cent. South Univ. 2015, 22, 103–113. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Chaba, J.M.; Nomngongo, P.N. Preparation of V2O5-ZnO coated carbon nanofibers: Application for removal of selected antibiotics in environmental matrices. J. Water Process Eng. 2018, 23, 50–60. [Google Scholar] [CrossRef]
- Chieng, H.I.; Zehra, T.; Lim, L.B.; Priyantha, N.; Tennakoon, D. Sorption characteristics of peat of Brunei Darussalam IV: Equilibrium, thermodynamics and kinetics of adsorption of methylene blue and malachite green dyes from aqueous solution. Environ. Earth Sci. 2014, 72, 2263–2277. [Google Scholar] [CrossRef]
- Benzaoui, T.; Selatnia, A.; Djabali, D. Adsorption of copper (II) ions from aqueous solution using bottom ash of expired drugs incineration. Adsorpt. Sci. Technol. 2018, 36, 114–129. [Google Scholar] [CrossRef]
- Basu, S.; Ghosh, G.; Saha, S. Adsorption characteristics of phosphoric acid induced activation of bio-carbon: Equilibrium, kinetics, thermodynamics and batch adsorber design. Process Saf. Environ. 2018, 117, 125–142. [Google Scholar] [CrossRef]
- Kazak, O.; Eker, Y.R.; Akin, I.; Bingol, H.; Tor, A. Green preparation of a novel red mud@carbon composite and its application for adsorption of 2,4-dichlorophenoxyacetic acid from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 23057–23068. [Google Scholar] [CrossRef]
- Vafajoo, L.; Cheraghi, R.; Dabbagh, R.; McKay, G. Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: Equilibrium, thermodynamic, and dynamic studies. Chem. Eng. J. 2018, 331, 39–47. [Google Scholar] [CrossRef]
- Vieira, R.M.; Vilela, P.B.; Becegato, V.A.; Paulino, A.T. Chitosan-based hydrogel and chitosan/acid-activated montmorillonite composite hydrogel for the adsorption and removal of Pb+2 and Ni+2 ions accommodated in aqueous solutions. J. Environ.Chem. Eng. 2018, 6, 2713–2723. [Google Scholar] [CrossRef]
- Tanzifi, M.; Hosseini, S.H.; Kiadehi, A.D.; Olazar, M.; Karimipour, K.; Rezaiemehr, R.; Ali, I. Artificial neural network optimization for methyl orange adsorption onto polyaniline nano-adsorbent: Kinetic, isotherm and thermodynamic studies. J. Mol. Liq. 2017, 244, 189–200. [Google Scholar] [CrossRef]
- Lin, G.; Hu, T.; Wang, S.; Xie, T.; Zhang, L.; Cheng, S.; Fu, L.; Xiong, C. Selective removal behavior and mechanism of trace Hg (II) using modified corn husk leaves. Chemosphere 2019, 225, 65–72. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Bhatnagar, A.; Vahala, R. Synthesis, characterization and exploitation of nano-TiO2/feldspar-embedded chitosan beads towards UV-assisted adsorptive abatement of aqueous arsenic (As). Chem. Eng. J. 2017, 316, 370–382. [Google Scholar] [CrossRef]
- Bentahar, Y.; Hurel, C.; Draoui, K.; Khairoun, S.; Marmier, N. Adsorptive properties of Moroccan clays for the removal of arsenic (V) from aqueous solution. Appl. Clay Sci. 2016, 119, 385–392. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Zhang, G.; Chen, J.P. Adsorptive removal of arsenic from water by an iron–zirconium binary oxide adsorbent. J. Colloid Interf. Sci. 2011, 358, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Anirudhan, T.; Nima, J.; Sandeep, S.; Ratheesh, V. Development of an amino functionalized glycidylmethacrylate-grafted-titanium dioxide densified cellulose for the adsorptive removal of arsenic (V) from aqueous solutions. Chem. Eng. J. 2012, 209, 362–371. [Google Scholar] [CrossRef]
- Javanbakht, V.; Ghoreishi, S.M.; Habibi, N.; Javanbakht, M. A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb (II) ions from aqueous solution. Powder Technol. 2016, 302, 372–383. [Google Scholar] [CrossRef]
- Postai, D.L.; Demarchi, C.A.; Zanatta, F.; Melo, D.C.C.; Rodrigues, C.A. Adsorption of rhodamine B and methylene blue dyes using waste of seeds of Aleurites Moluccana, a low cost adsorbent. Alexandria Eng. J. 2016, 55, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. Kinetics and thermodynamics of Cd (II) biosorption onto loquat (Eriobotrya japonica) leaves. J. Saudi Chem. Soc. 2014, 18, 486–493. [Google Scholar] [CrossRef]
- Mokkapati, R.; Ratnakaram, V.; Mokkapati, J. Utilization of agro-waste for removal of toxic hexavalent chromium: Surface interaction and mass transfer studies. Int. J. Environ. Sci. Technol. 2018, 15, 875–886. [Google Scholar] [CrossRef]
- Le, N.C.; Van Phuc, D. Sorption of lead (II), cobalt (II) and copper (II) ions from aqueous solutions by γ-MnO2 nanostructure. Adv. Nat. Sci: Nanosci. Nanotechnol. 2015, 6, 025014. [Google Scholar] [CrossRef]
- Valetti, N.W.; Picó, G. Adsorption isotherms, kinetics and thermodynamic studies towards understanding the interaction between cross-linked alginate-guar gum matrix and chymotrypsin. J. Chromatogr. B 2016, 1012, 204–210. [Google Scholar] [CrossRef]
- Shahbeig, H.; Bagheri, N.; Ghorbanian, S.A.; Hallajisani, A.; Poorkarimi, S. A new adsorption isotherm model of aqueous solutions on granular activated carbon. World J. Model. Simul. 2013, 9, 243–254. [Google Scholar]
Sample Availability: No compound is available from the authors. |




| Standard Run | pH | MA | ET | %RE |
|---|---|---|---|---|
| 1 | 2 | 50 | 5 | 98.8 |
| 2 | 8 | 50 | 5 | 32.4 |
| 3 | 2 | 200 | 5 | 98.9 |
| 4 | 8 | 200 | 5 | 47.8 |
| 5 | 2 | 50 | 40 | 99.4 |
| 6 | 8 | 50 | 40 | 52.5 |
| 7 | 2 | 200 | 40 | 98.5 |
| 8 | 8 | 200 | 40 | 51.3 |
| 9 (C) | 5 | 125 | 22.5 | 68.7 |
| 10 (C) | 5 | 125 | 22.5 | 68.4 |
| 11 (C) | 5 | 125 | 22.5 | 68.4 |
| 12 (C) | 5 | 125 | 22.5 | 69.6 |
| 13 (C) | 5 | 125 | 22.5 | 68.9 |
| 14 (C) | 5 | 125 | 22.5 | 67.6 |
| 15 (C) | 5 | 125 | 22.5 | 68.7 |
| 16 (C) | 5 | 125 | 22.5 | 67.2 |
| Standard Run | pH | ET (min) | %RE |
|---|---|---|---|
| 1 | 2.0 | 5.0 | 99.1 |
| 2 | 2.0 | 40.0 | 99.3 |
| 3 | 8.0 | 5.0 | 44.4 |
| 4 | 8.0 | 40.0 | 52.4 |
| 5 | 0.8 | 22.5 | 57.3 |
| 6 | 9.2 | 22.5 | 46.8 |
| 7 | 5.0 | −2.2 | 7.1 |
| 8 | 5.0 | 47.2 | 98.6 |
| 9 (C) | 5.0 | 22.5 | 91.2 |
| 10 (C) | 5.0 | 22.5 | 93.0 |
| 11 (C) | 5.0 | 22.5 | 89.1 |
| 12 (C) | 5.0 | 22.5 | 90.4 |
| 13 (C) | 5.0 | 22.5 | 89.4 |
| 14 (C) | 5.0 | 22.5 | 92.9 |
| 15 (C) | 5.0 | 22.5 | 90.7 |
| 16 (C) | 5.0 | 22.5 | 88.3 |
| 17 (C) | 5.0 | 22.5 | 90.2 |
| 18 (C) | 5.0 | 22.5 | 90.0 |
| Isotherms | Parameters | R2 | |
|---|---|---|---|
| Langmuir | qmax (mg/g) | 27.8 | 0.9922 |
| KL (L μg−1) | 4.0 | ||
| RL | 0.021–0.26 | ||
| Freundlich | KF (L g−1) | 0.42 | 0.9706 |
| n | 3 | ||
| Temkin | KT (L g−1) | 0.018 | 0.9 |
| bT (kJ mol−1) | 833 | ||
| B | 2.974 | ||
| Redlich-Peterson | KRP (L g−1) | 1150 | 0.9909 |
| β | 0.98 | ||
| α | 81.9 | ||
| Sips | KS (L g−1) | 0.93 | 0.9907 |
| n | 1.05 | ||
| qmax (mg g−1) | 28.2 | ||
| Hill | qH (mg g−1) | 29.3 | 0.9912 |
| KD | 0.78 | ||
| nH | 0.86 | ||
| Adsorbent | Adsorption Capacity (mg/g) | pH | Refs | |
|---|---|---|---|---|
| As (III) and As (V) | CeO2/Fe2O3/graphene nanocomposite | 84–101 | 7.8 | [29] |
| As | nano-TiO2/feldspar-embedded chitosan beads | 3–6 | 4–10 | [44] |
| As(III) and As(V) | zeolitic imidazolate framework-8 (ZIF-8) | 50–60 | 7 | [46] |
| As(V) | Moroccan clays | 0.56–1.1 | 7 | [45] |
| As(V) and As(III) | iron–zirconium (Fe–Zr) binary oxide | 46 and 120 | 7 | [47] |
| As(V) | amino functionalized glycidylmethacrylate-grafted-titanium dioxide densified cellulose | 109 | 6 | [48] |
| As | MWCNT-Fe3O4@Zeo | 28 | 2.9 | Current Study |
| Samples | Initial (mg L−1) | After Adsorption (µg L−1) | %RE |
|---|---|---|---|
| AMD 1 | 7.02 ± 0.12 | 13.9 ± 0. 9 | 99.8 ± 1.2 |
| AMD 2 | 5.24 ± 0.22 | 9.60 ± 0.31 | 99.8 ± 0.9 |
| ADM 3 | 14.5 ± 0.9 | 957 ± 5 | 93.4 ± 1.3 |
| AMD 4 | 7.52 ± 0.21 | 91.2 ± 1.1 | 98.7 ± 1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugushe, A.S.; Nqombolo, A.; Nomngongo, P.N. Application of Response Surface Methodology and Desirability Function in the Optimization of Adsorptive Remediation of Arsenic from Acid Mine Drainage Using Magnetic Nanocomposite: Equilibrium Studies and Application to Real Samples. Molecules 2019, 24, 1792. https://doi.org/10.3390/molecules24091792
Gugushe AS, Nqombolo A, Nomngongo PN. Application of Response Surface Methodology and Desirability Function in the Optimization of Adsorptive Remediation of Arsenic from Acid Mine Drainage Using Magnetic Nanocomposite: Equilibrium Studies and Application to Real Samples. Molecules. 2019; 24(9):1792. https://doi.org/10.3390/molecules24091792
Chicago/Turabian StyleGugushe, Aphiwe Siyasanga, Azile Nqombolo, and Philiswa N. Nomngongo. 2019. "Application of Response Surface Methodology and Desirability Function in the Optimization of Adsorptive Remediation of Arsenic from Acid Mine Drainage Using Magnetic Nanocomposite: Equilibrium Studies and Application to Real Samples" Molecules 24, no. 9: 1792. https://doi.org/10.3390/molecules24091792