Asymmetric Henry Reaction of 2-Acylpyridine N-Oxides Catalyzed by a Ni-Aminophenol Sulfonamide Complex: An Unexpected Mononuclear Catalyst
Abstract
:1. Introduction
2. Results and Discussion
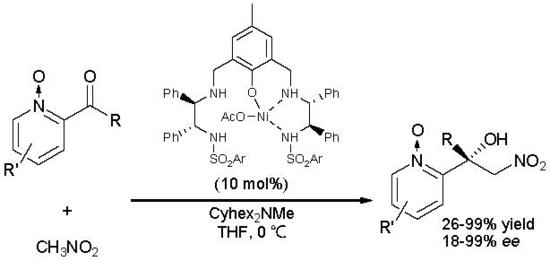
2.1. Catalytic Asymmetric Henry Reaction
2.2. Mechanistic Studies of Ni-Aminophenol Sulfonamide Complex
3. Experimental Section
3.1. General Information
3.2. General Procedure for Catalytic Asymmetric Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christoffers, J.; Mann, A. Enantioselective Construction of Quaternary Stereocenters. Angew. Chem. Int. Ed. 2001, 40, 4591–4597. [Google Scholar] [CrossRef]
- Douglas, C.J.; Overman, L.E. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc. Natl. Acad. Sci. USA 2004, 101, 5363–5367. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M.; Jiang, C. Catalytic Enantioselective Construction of All-Carbon Quaternary Stereocenters. Synthesis 2006, 369–396. [Google Scholar] [CrossRef]
- Das, J.P.; Marek, I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 2011, 47, 4593–4623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, S.J.; Liu, W.B.; Stoltz, B.M. Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res. 2015, 48, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Rivas, F. All-carbon quaternary centers in natural products and medicinal chemistry: Recent advances. Tetrahedron 2016, 72, 6729–6777. [Google Scholar] [CrossRef]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Mielgo, A. Unveiling Reliable Catalysts for the Asymmetric Nitroaldol (Henry) Reaction. Angew. Chem. Int. Ed. 2004, 43, 5442–5444. [Google Scholar] [CrossRef]
- Boruwa, J.; Gogoi, N.; Saikia, P.P.; Barua, N.C. Catalytic asymmetric Henry reaction. Tetrahedron Asymmetry 2006, 17, 3315–3326. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Laso, A. Recent Advances in the Catalytic Asymmetric Nitroaldol (Henry) Reaction. Eur. J. Org. Chem. 2007, 2561–2574. [Google Scholar] [CrossRef]
- Blay, G.; Hernández-Olmos, V.; Pedro, J.R. Development of New N,N-Ligands for the Enantioselective Copper(II)-Catalyzed Henry Reaction. Synlett 2011, 2011, 1195–1211. [Google Scholar] [CrossRef]
- Milner, S.E.; Moody, T.S.; Maguire, A.R. Biocatalytic Approaches to the Henry (Nitroaldol) Reaction. Eur. J. Org. Chem. 2012, 2012, 3059–3067. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Xu, Y.; Wang, Z. Recent progress in copper catalyzed asymmetric Henry reaction. Chin. Chem. Lett. 2018, 29, 873–883. [Google Scholar] [CrossRef]
- Tur, F.; Saá, J.M. Direct, Catalytic Enantioselective Nitroaldol (Henry) Reaction of Trifluoromethyl Ketones: An Asymmetric Entry to α-Trifluoromethyl-Substituted Quaternary Carbons. Org. Lett. 2007, 9, 5079–5082. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Sinisi, R.; Umani-Ronchi, A. Enantioselective organocatalyzed Henry reaction with fluoromethyl ketones. Chem. Commun. 2008, 4360–4362. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wolf, C. Synthesis of chiral tertiary trifluoromethyl alcohols by asymmetric nitroaldol reaction with a Cu(II)-bisoxazolidine catalyst. Chem. Commun. 2010, 46, 8026–8028. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.; Connon, S.J. A New Class of Urea-Substituted Cinchona Alkaloids Promote Highly Enantioselective Nitroaldol reactions of Trifluoromethylketones. Org. Lett. 2011, 13, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Kumagai, N.; Shibasaki, M. Heterogeneous Heterobimetallic Catalysis Enabling Expeditious Access to CF3-Containing vic-Amino Alcohols. Org. Lett. 2018, 20, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.; Juhl, K.; Hazell, R.G.; Jørgensen, K.A. Catalytic asymmetric Henry reactions—A simple approach to optically active β-nitro α-hydroxy esters. Chem. Commun. 2001, 2222–2223. [Google Scholar] [CrossRef]
- Choudary, B.M.; Ranganath, K.V.S.; Pal, U.; Kantam, M.L.; Sreedhar, B. Nanocrystalline MgO for Asymmetric Henry and Michael Reactions. J. Am. Chem. Soc. 2005, 127, 13167–13171. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Deng, L. Enantioselective Nitroaldol Reaction of α-Ketoesters Catalyzed by Cinchona Alkaloids. J. Am. Chem. Soc. 2006, 128, 732–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, B.; Liu, X.; Huang, J.; Wen, Y.; Feng, X. Highly Enantioselective Henry (Nitroaldol) Reaction of Aldehydes and α-Ketoesters Catalyzed by N,N’-Dioxide-Copper(I) Complexes. J. Org. Chem. 2007, 72, 9323–9328. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, G.; Yang, J.; Liang, G.; Deng, P.; Xiong, Y.; Zhou, H. Catalytic enantioselective Henry reaction of α-keto esters, 2-acylpyridines and 2-acylpyridine N-oxides. RSC Adv. 2018, 8, 9414–9422. [Google Scholar] [CrossRef]
- Karasawa, T.; Orize, R.; Kumagai, N.; Shibasaki, M. anti-Selective Catalytic Asymmetric Nitroaldol Reaction of α-Keto Esters: Intriguing Solvent Effect, Flow Reaction, and Synthesis of Active Pharmaceutical Ingredients. J. Am. Chem. Soc. 2018, 140, 12290–12295. [Google Scholar] [CrossRef]
- Prathima, P.S.; Srinivas, K.; Balaswamy, K.; Arundhathi, R.; Reddy, G.N.; Sridhar, B.; Rao, M.M.; Likhar, P.R. Biscinchona alkaloid catalysed Henry reaction of isatins: Enantioselective synthesis of 3-hydroxy-3-(nitromethyl)indolin-2-ones. Tetrahedron Asymmetry 2011, 22, 2099–2103. [Google Scholar] [CrossRef]
- Xu, H.; Wolf, C. Asymmetric Synthesis of Chiral 1,3-Diaminopropanols: Bisoxazolidine-Catalyzed C-C Bond Formation with α-Keto Amides. Angew. Chem. Int. Ed. 2011, 50, 12249–12252. [Google Scholar] [CrossRef]
- Mandal, T.; Samanta, S.; Zhao, C.-G. Organocatalytic Highly Enantioselective Nitroaldol Reaction of α-Ketophosphonates and Nitromethane. Org. Lett. 2007, 9, 943–945. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Zhu, Y.; Shang, D.; Gao, B.; Liu, X.; Feng, X.; Su, Z.; Hu, C. A Secondary Amine Amide Organocatalyst for the Asymmetric Nitroaldol Reaction of α-Ketophosphonates. Chem. Eur. J. 2008, 14, 10896–10899. [Google Scholar] [CrossRef]
- Blay, G.; Hernández-Olmos, V.; Pedro, J.R. The Construction of Quaternary Stereocenters by the Henry Reaction: Circumventing the Usual Reactivity of Substituted Glyoxals. Chem. Eur. J. 2011, 17, 3768–3773. [Google Scholar] [CrossRef]
- Tosaki, S.; Hara, K.; Gnanadesikan, V.; Morimoto, H.; Harada, S.; Sugita, M.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Mixed La−Li Heterobimetallic Complexes for Tertiary Nitroaldol Resolution. J. Am. Chem. Soc. 2006, 128, 11776–11777. [Google Scholar] [CrossRef]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Müller, W.E.G.; Van Soest, R.W.M. Four New Bioactive Manzamine-Type Alkaloids from the Philippine Marine Sponge Xestospongia ashmorica. J. Nat. Prod. 1996, 59, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three New Manzamine Alkaloids from a Common Indonesian Sponge and Their Activity against Infectious and Tropical Parasitic Diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tsukano, C.; Kwon, E.; Takemoto, Y.; Hirama, M. Total Syntheses of Complanadines A and B. Angew. Chem. Int. Ed. 2013, 52, 1722–1725. [Google Scholar] [CrossRef]
- Chelucci, G.; Murineddu, G.; Pinna, G.A. Chiral pyridine N-oxides: Useful ligands for asymmetric catalysis. Tetrahedron Asymmetry 2004, 15, 1373–1389. [Google Scholar] [CrossRef]
- Halcrow, M.A. The synthesis and coordination chemistry of 2,6-bis(pyrazolyl)pyridines and related ligands -Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Chelucci, G. Synthesis and application in asymmetric catalysis of camphor-based pyridine ligands. Chem. Soc. Rev. 2006, 35, 1230–1243. [Google Scholar] [CrossRef]
- Kwong, H.L.; Yeung, H.L.; Yeung, C.T.; Lee, W.S.; Wong, W.L. Chiral pyridine-containing ligands in asymmetric catalysis. Coord. Chem. Rev. 2007, 251, 2188–2222. [Google Scholar] [CrossRef]
- Chen, J.; Takenaka, N. Helical Chiral Pyridine N-Oxides: A New Family of Asymmetric Catalysts. Chem. Eur. J. 2009, 15, 7268–7276. [Google Scholar] [CrossRef] [PubMed]
- Chelucci, G. Metal-complexes of optically active amino- and imino-based pyridine ligands in asymmetric catalysis. Coord. Chem. Rev. 2013, 257, 1887–1932. [Google Scholar] [CrossRef]
- Holmquist, M.; Blay, G.; Muñoz, M.C.; Pedro, J.R. Enantioselective Addition of Nitromethane to 2-Acylpyridine N-Oxides. Expanding the Generation of Quaternary Stereocenters with the Henry Reaction. Org. Lett. 2014, 16, 1204–1207. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, D.; Qin, B.; Hou, Z.; Liu, X.; Feng, X. Highly Enantioselective Aza-Henry Reaction of N-Tosyl Imines Catalyzed by N,N’-Dioxide−Cu(I) Complexes. J. Org. Chem. 2007, 72, 10302–10304. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Liu, X.; Wang, L.; Wang, J.; Feng, X. Highly Enantioselective Aza-Henry Reaction of Ketoimines Catalyzed by Chiral N,N’-Dioxide−Copper(I) Complexes. Org. Lett. 2008, 10, 5305–5308. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Xiao, X.; Zhao, X.; Liu, X.; Lin, L.; Feng, X. Catalytic Asymmetric Henry Reaction of Nitroalkanes and Aldehydes Catalyzed by a Chiral N,N’-Dioxide/Cu(I) Complex. J. Org. Chem. 2015, 80, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liang, G.; Wang, Y.; Deng, P.; Zhou, H. A homodinuclear cobalt complex for the catalytic asymmetric Michael reaction of β-ketoesters to nitroolefins. Org. Biomol. Chem. 2018, 16, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, P.; Zhou, J.; Liu, M.; Liang, G.; Xiong, Y.; Zhou, H. A novel homobimetallic nickel complex for the asymmetric direct Mannich reaction of imines: A practical method on a multi-gram scale. Chem. Commun. 2017, 53, 12914–12917. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, P.; Zeng, Y.; Xiong, Y.; Zhou, H. anti-Selective Asymmetric Henry Reaction Catalyzed by a Heterobimetallic Cu−Sm−Aminophenol Sulfonamide Complex. Org. Lett. 2016, 18, 1578–1581. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Chiral N,N’-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions. Acc. Chem. Res. 2011, 44, 574–587. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Chiral N,N’-dioxide ligands: Synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014, 1, 298–302. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, H.; Xia, Y.; Feng, X. Asymmetric Cycloaddition and Cyclization Reactions Catalyzed by Chiral N,N’-Dioxide–Metal Complexes. Acc. Chem. Res. 2017, 50, 2621–2631. [Google Scholar] [CrossRef]
- Meyer, F.; Kozlowski, H. Nickel. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Mever, T.J., Eds.; Elsevier: Oxford, UK, 2003; Volume 6, pp. 247–554. [Google Scholar]
- Evans, D.A.; Seidel, D.; Rueping, M.H.; Lam, W.; Shaw, J.T.; Downey, C.W. A New Copper Acetate-Bis(oxazoline)-Catalyzed Enantioselective Henry Reaction. J. Am. Chem. Soc. 2003, 125, 12692–12693. [Google Scholar] [CrossRef]
Sample Availability: All data generated and/or compound samples analyzed during this study are included in this article and are available from the corresponding author on reasonable request. |





 | |||||
| Entry | Ni(OAc)2 (x) | Ligand (y) | x/y | Yield (%) b | ee (%) c |
| 1 | 20 | L1 (10) | 2/1 | 94 | 76 |
| 2 | 15 | L1 (10) | 1.5/1 | 98 | 81 |
| 3 | 10 | L1 (10) | 1/1 | 81 | 81 |
| 4 | 10 | L1 (11) | 1/1.1 | 86 | 85 |
| 5 | 10 | L1 (12) | 1/1.2 | 79 | 83 |
| 6 | 10 | L1 (15) | 1/1.5 | 72 | 84 |
| 7 | 10 | L1 (20) | 1/2 | 73 | 83 |
| 8 | 10 | L2 (11) | 1/1.1 | 99 | 91 |
| 9 | 10 | L3 (11) | 1/1.1 | 90 | 83 |
| 10 | 10 | L4 (11) | 1/1.1 | 91 | 76 |
| 11 | 10 | L5 (11) | 1/1.1 | 89 | 89 |
| 12 | 10 | L6 (11) | 1/1.1 | 50 | 4 d |
| 13 | 10 | L7 (11) | 1/1.1 | 82 | 76 |
| 14 | 10 | L8 (11) | 1/1.1 | 92 | 92 |
| 15 | 10 | L9 (11) | 1/1.1 | 88 | 7 |
| 16 | 10 | L10 (11) | 1/1.1 | 69 | 5 d |
| 17 | 10 | L11 (11) | 1/1.1 | 98 | 15 |
 | |||
| Entry | Base | Yield (%) b | ee (%) c |
| 1 | iPr2NEt | 99 | 91 |
| 2 | Cyhex2NMe d | 99 | 94 |
| 3 | Et3N | 99 | 89 |
| 4 | NMM e | 87 | 86 |
| 5 | iPr2NH | 99 | 81 |
| 6 | Bu2NH | 99 | 88 |
| 7 | Cyhex2NH f | 99 | 88 |
| 8 g | Cyhex2NMe d | 99 | 83 |
 | ||||||
| Entry | R1 | R2 | Product | Time (h) | Yield (%) b | ee (%) c |
| 1 | H | Me | 2a | 15 | 99 | 94 |
| 2 | 4-Me | Me | 2b | 15 | 99 | 99 |
| 3 | 5-Me | Me | 2c | 15 | 99 | 97 |
| 4 | 6-Me | Me | 2d | 24 | 99 | 17 |
| 5 | 4-Cl | Me | 2e | 17 | 99 | 92 |
| 6 | 5-Br | Me | 2f | 72 | 26 | 84 |
| 7 | H | Et | 2g | 42 | 86 | 92 |
| 8 | H | Bu | 2h | 42 | 67 | 69 |
| 9 | H | 4-ClC6H4 | 2i | 72 | 48 | 79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Gui, D.; Deng, P.; Zhou, H. Asymmetric Henry Reaction of 2-Acylpyridine N-Oxides Catalyzed by a Ni-Aminophenol Sulfonamide Complex: An Unexpected Mononuclear Catalyst. Molecules 2019, 24, 1471. https://doi.org/10.3390/molecules24081471
Liu M, Gui D, Deng P, Zhou H. Asymmetric Henry Reaction of 2-Acylpyridine N-Oxides Catalyzed by a Ni-Aminophenol Sulfonamide Complex: An Unexpected Mononuclear Catalyst. Molecules. 2019; 24(8):1471. https://doi.org/10.3390/molecules24081471
Chicago/Turabian StyleLiu, Mouxiong, Dongdong Gui, Ping Deng, and Hui Zhou. 2019. "Asymmetric Henry Reaction of 2-Acylpyridine N-Oxides Catalyzed by a Ni-Aminophenol Sulfonamide Complex: An Unexpected Mononuclear Catalyst" Molecules 24, no. 8: 1471. https://doi.org/10.3390/molecules24081471