The Morphology and Performance of Poly(Vinyl Chloride) Containing Melamine Schiff Bases against Ultraviolet Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Schiff Bases 1−5
2.2. Energy Dispersive X-Ray Spectroscopy
2.3. FTIR Spectroscopy
2.4. Weight Loss
2.5. Viscosity Average Molecular Weight
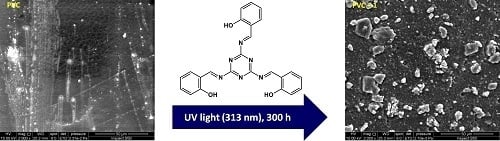
2.6. Microscopic Surface Morphology
2.7. Scanning Electron Microscopy
2.8. Atomic Force Microscopy
3. Experimental
3.1. General
3.2. Synthesis of Schiff Bases 1–5
3.3. Preparation of PVC Films
3.4. Accelerated Ultraviolet Weathering Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Keneghan, B.; Egan, L. Plastics: Looking at the Future and Learning from the Past; Archetype Publications Ltd.: London, UK, 2008. [Google Scholar]
- Shashoua, Y. Conservation of Plastics: Material Science: Degradation and Preservation; Butterworth-Heinemann: London, UK, 2008. [Google Scholar]
- Huang, Z.; Ding, A.; Guo, H.; Lu, G.; Huang, X. Construction of nontoxic polymeric UV-absorber with great resistance to UV-photoaging. Sci. Rep. 2016, 6, 25508. [Google Scholar] [CrossRef] [PubMed]
- Mango, L.A.; Lenz, R.W. Hydrogenation of unsaturated polymers with diimide. Makromol. Chem. 1973, 163, 13–36. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC wastes. Polym. Degrad. Stabil. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Goodman, S. The Microwave Induced Pyrolysis of Problematic Plastics Enabling Recovery and Component Reuse. Ph.D. Thesis, Imperial College, London, UK, 2014. [Google Scholar]
- Baishya, P.; Mahanta, D.K. Improvised segregation of recyclable materials in Guwahati city, India: A case study. Clarion J. 2013, 2, 46–52. [Google Scholar]
- Mallakpour, S.; Sadeghzadeh, R. A benign and simple strategy for surface modification of Al2O3 nanoparticles with citric acid and L(+)-ascorbic acid and its application for the preparation of novel poly(vinyl chloride) nanocomposite films. Adv. Polym. Techol. 2017, 36, 409–417. [Google Scholar] [CrossRef]
- Sterzyński, T.; Tomaszewska, J.; Piszczek, K.; Skórczewska, K. The influence of carbon nanotubes on the PVC glass transition temperature. Compos. Sci. Technol. 2010, 70, 966–969. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N.; Jawad, A.; Win, Y.-F. New stabilizers for PVC based on some diorganotin(IV) complexes with bezamidoleucine. Arab. J. Chem. 2016, 9, S1394–S1401. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Hazra, S.; Guedes de Silva, M.F.C.; Pombeiro, A.J.L. A sulfonated Schiff base dimethyltin(IV) coordination polymer: Synthesis, characterization and application as a catalyst for ultrasound- or microwave-assisted Baeyer-Villiger oxidation under solvent-free conditions. RSC Adv. 2016, 6, 78225–78233. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizng efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mikhael, M.G.; Mohamed, N.A.; Yassin, A.A. N-substituted maleimides as thermal stabilizers for rigid poly (vinyl chloride). Angew. Makromol. Chem. 1989, 168, 23–25. [Google Scholar] [CrossRef]
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl Chloride) Photostabilization in the Presence of Schiff Bases Containing a Thiadiazole Moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M. PVC Additives: Performance, Chemistry, Developments, and Sustainability; Carl Hanser Verlag: Munich, Germany, 2015. [Google Scholar]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Yang, T.-C.; Noguchi, T.; Isshiki, M.; Wu, J.-H. Effect of titanium dioxide particles on the surface morphology and the mechanical properties of PVC composites during QUV accelerated weathering. Polym. Compos. 2016, 37, 3391–3397. [Google Scholar] [CrossRef]
- Yang, T.-C.; Noguchi, T.; Isshiki, M.; Wu, J.-H. Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering. Polym. Degrad. Stab. 2014, 104, 33–39. [Google Scholar] [CrossRef]
- Sokhandani, P.; Babaluo, A.A.; Rezaei, M.; Shahrezaei, M.; Hasanzadeh, A.; Mehmandoust, S.G.; Mehdizadeh, R. Nanocomposites of PVC/TiO2 nanorods: Surface tension and mechanical properties before and after UV exposure. J. Appl. Polym. Sci. 2013, 129, 3265–3272. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, S.; Tiwari, N.; Bahadur, I.; Ebenso, E.E. Experimental and quantum chemical studies of synthesized triazine derivatives as an efficient corrosion inhibitor for N80 steel in acidic medium. J. Mol. Liq. 2015, 212, 151–167. [Google Scholar] [CrossRef]
- Hu, Z.; Meng, Y.; Ma, X.; Zhu, H.; Li, J.; Li, C.; Cao, D. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1M HCl. Corros. Sci. 2016, 112, 563–575. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Xu, J.; Wu, D. Incorporating fluorescent dyes into monodisperse melamine-formaldehyde resin microspheres via an organic sol-gel process: A pre-polymer doping strategy. J. Mater. Chem. B 2014, 2, 5837–5846. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; El-Hiti, G.A.; Hashim, H.; Hameed, A.S.; Ahmed, D.S.; Yousif, E. SEM analysis of the tunable honeycomb structure of irradiated poly(vinyl chloride) films doped with polyphosphate. Heliyon 2018, 4, e01013. [Google Scholar] [CrossRef] [PubMed]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of novel ball-like polystyrene films containing Schiff bases microspheres as photostabilizers. Polymers 2018, 10, 1185. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Synthesis and characterization of a new photochromic alkylene sulfide derivative. J. Sulfur Chem. 2018, 39, 182–192. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus. equi using different carbon sources. Arab. J. Sci. Eng. 2017, 42, 2371–2379. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl) propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Kaya, I.; Yildirim, M. Synthesis and characterization of graft copolymers of melamine: Thermal stability, electrical conductivity, and optical properties. Synth. Met. 2009, 159, 1572–1582. [Google Scholar] [CrossRef]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(viny1 chloride). 1. A reexamination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Scott, G. Mechanism of Polymer Degradation and Stabilization; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates on the photooxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chloride. J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef]
- Torikai, A.; Hasegawa, H. Accelerated photodegradation of poly(vinyl chloride). Polym. Degrad. Stab. 1999, 63, 441–445. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Skillicorn, D.E.; Perkins, G.G.A.; Slark, A.; Dawkins, J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: New York, NY, USA, 1978. [Google Scholar]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride) (part 1). Macromol. Mater. Eng. 1977, 64, 89–99. [Google Scholar] [CrossRef]
- Kayyarapu, B.; Kumar, M.Y.; Mohommad, H.B.; Neeruganti, G.O.; Chekuri, R. Structural, thermal and optical properties of pure and Mn2+ doped poly(vinyl chloride) films. Mater. Res. 2016, 19, 1167–1175. [Google Scholar] [CrossRef]
- Devi, M.R.; Saranya, A.; Pandiarajan, J.; Dharmaraja, J.; Prithivikumaran, N.; Jeyakumaran, N. Fabrication, spectral characterization, XRD and SEM studies on some organic acids doped polyaniline thin films on glass substrate. JKSUS 2018, in press. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.-M.; Jiang, G.-D. Different photodegradation processes of PVC with different average degrees of polymerization. J. Appl. Polym. Sci. 2008, 107, 528–540. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603–604, 616–626. [Google Scholar] [CrossRef] [PubMed]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. Appl. Polym. 2003, 89, 36–46. [Google Scholar] [CrossRef]
Sample Availability: Samples of Schiff bases are available from the authors upon request. |












| Additive | Ar | Yield (%) | Melting Point (°C) | Calcd. (Found; %) | ||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 1 | 2-HOC6H4 | 71 | 293−295 | 65.75 (66.03) | 4.14 (4.34) | 19.17 (19.03) |
| 2 | 3-HOC6H4 | 78 | 288−289 | 65.75 (65.94) | 4.14 (4.31) | 19.17 (19.22) |
| 3 | 4-HOC6H4 | 73 | 274−275 | 65.75 (65.95) | 4.14 (4.24) | 19.17 (19.19) |
| 4 | 4-NO2C6H4 | 76 | 266−267 | 54.86 (54.89) | 2.88 (2.97) | 23.99 (24.07) |
| 5 | C6H5CH=CH | 70 | 274−275 | 76.90 (77.13) | 5.16 (5.91) | 17.94 (17.79) |
| Stabilizer | FR-IR (υ, cm−1) | |||
|---|---|---|---|---|
| OH | CH=N | C=N (Ar) | C=C (Ar) | |
| 1 | 3332 | 1632 | 1539 | 1465 |
| 2 | 3338 | 1639 | 1546 | 1462 |
| 3 | 3335 | 1643 | 1543 | 1438 |
| 4 | - | 1639 | 1543 | 1458 |
| 5 | - | 1647 | 1543 | 1465 |
| Stabilizer | 1H-NMR (400 MHz: DMSO-d6, ppm) |
|---|---|
| 1 | 11.22 (br. s, exch., 3 H, OH), 8.49 (s, 3 H, CH=N), 7.61 (d, J = 8.2 Hz, 3 H, Ar), 7.01 (t, J = 8.2 Hz, 3 H, Ar), 6.58 (d, J = 8.2 Hz, 3 H, Ar), 5.99 (s, 3 H, Ar) |
| 2 | 11.15 (br. s, exch., 3 H, OH), 9.29 (s, 3 H, CH=N), 7.45–7.33 (m, 9 H, Ar), 7.15 (d, J = 8.4 Hz, 3 H, Ar) |
| 3 | 10.36 (br. s, exch., 3 H, OH), 9.26 (s, 3 H, CH=N), 7.75 (d, J = 8.2 Hz, 6 H, Ar), 6.90 (d, J = 8.2 Hz, 6 H, Ar) |
| 4 | 9.28 (s, 3 H, CH=N), 8.44–8.14 (m, 12 H, Ar) |
| 5 | 9.28 (s, 3 H, CH=N), 7.95–7.55 (m, 9 H, Ar), 7.49–7.42 (m, 9 H, CH and Ar), 6.68 (d, J = 8.0 Hz, 3 H, CH) |
| Irradiated PVC (300 h) | Rq |
|---|---|
| PVC (blank) | 159.5 |
| PVC + 1 | 15.5 |
| PVC + 2 | 24.3 |
| PVC + 3 | 27.5 |
| PVC + 4 | 29.1 |
| PVC + 5 | 26.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The Morphology and Performance of Poly(Vinyl Chloride) Containing Melamine Schiff Bases against Ultraviolet Light. Molecules 2019, 24, 803. https://doi.org/10.3390/molecules24040803
El-Hiti GA, Alotaibi MH, Ahmed AA, Hamad BA, Ahmed DS, Ahmed A, Hashim H, Yousif E. The Morphology and Performance of Poly(Vinyl Chloride) Containing Melamine Schiff Bases against Ultraviolet Light. Molecules. 2019; 24(4):803. https://doi.org/10.3390/molecules24040803
Chicago/Turabian StyleEl-Hiti, Gamal A., Mohammad Hayal Alotaibi, Ahmed A. Ahmed, Basheer A. Hamad, Dina S. Ahmed, Ahmed Ahmed, Hassan Hashim, and Emad Yousif. 2019. "The Morphology and Performance of Poly(Vinyl Chloride) Containing Melamine Schiff Bases against Ultraviolet Light" Molecules 24, no. 4: 803. https://doi.org/10.3390/molecules24040803