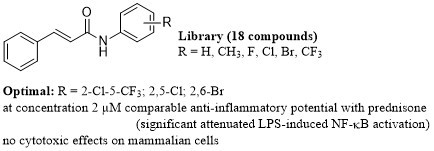
Investigation of Anti-Inflammatory Potential of N-Arylcinnamamide Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. X-Ray Crystallography
2.3. In Vitro Cell Viability Assay
2.4. Inhibition of NF-κB Activity and Cell Signaling In Vitro
2.5. Inhibition of TNF-α Secretion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. Synthesis
3.2. X-Ray Crystallography
3.3. Cell Cultivation
3.4. Cell Viability Determination
3.5. Determination of NF-κB Activity
3.6. Immunocytochemical Analysis of NF-κB Nuclear Migration
3.7. Signaling Pathway Analysis
3.8. Differentiation Monocytes into Macrophages and Evaluation of TNF-α Secretion
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Nestle, F.O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 2004, 113, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Jampilek, J.; Dolezal, M.; Opletalova, V.; Hartl, J. 5-Lipoxygenase, leukotrienes biosynthesis and potential antileukotrienic agents. Curr. Med. Chem. 2006, 13, 117–129. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.J.; Jampilek, J. Anti-infective drug discovery based on diversified plant natural compounds. Curr. Org. Chem. 2017, 21, 1775–1776. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents – a review. Curr Med Chem 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-Inflammatory activities of cinnamomum cassia constituents in vitro and in vivo. Evid-Based Compl. Alt. 2012, 2012, 429320. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Raines, R.T. An evaluation of peptide-bond isosteres. ChemBioChem 2011, 12, 1801–1807. [Google Scholar] [CrossRef] [Green Version]
- Mrozek-Wilczkiewicz, A.; Kalinowski, D.; Musiol, R.; Finster, J.; Szurko, A.; Serafin, K.; Knas, M.; Kamalapuram, S.K.; Kovacevic, Z.; Jampilek, J.; et al. Investigating anti-proliferative activity of styrylazanaphthalenes and azanaphthalenediones. Bioorg. Med. Chem. 2010, 18, 2664–2671. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]- benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef] [Green Version]
- Imramovsky, A.; Pesko, M.; Ferriz, J.M.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis—Inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Ferriz, J.M.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef]
- Mahesh, S.; Tang, K.C.; Raj, M. Amide bond activation of biological molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michnova, H.; Pospisilova, S.; Gonec, T.; Kapustikova, I.; Kollar, P.; Kozik, V.; Musiol, R.; Jendrzejewska, I.; Vanco, J.; Trávníček, Z.; et al. Bioactivity of methoxylated and methylated 1-hydroxynaphthalene-2-carboxanilides: Comparative molecular surface analysis. Molecules 2019, 24, 2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.Z.; Zhang, Y.L.; Liu, X.; Fang, Q.L.; Wang, Z.; Fu, L.L.; Liu, Z.G.; Wang, Y.; Zhao, Y.J.; Li, X.K.; et al. Discovery of a New inhibitor of myeloid differentiation 2 from cinnamamide derivatives with anti-inflammatory activity in sepsis and acute lung injury. J. Med. Chem. 2016, 59, 2436–2451. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure-activity relationships. Eur. J. Med. Chem. 2019, 181, 111561. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, S.; Kos, J.; Michnova, H.; Kapustikova, I.; Strharsky, T.; Oravec, M.; Moricz, A.M.; Bakonyi, J.; Kauerova, T.; Kollar, P.; et al. Synthesis and spectrum of biological activities of novel N-arylcinnamamides. Int. J. Mol. Sci. 2018, 19, 2318. [Google Scholar] [CrossRef] [Green Version]
- Pospisilova, S.; Kos, J.; Michnova, H.; Strharsky, T.; Cizek, A.; Jampilek, J. N-Arylcinnamamides as antistaphylococcal agents. In Proceedings of the 4th International Electronic Conference on Medicinal Chemistry (ECMC-4), 1–30 November 2018; Available online: https://sciforum.net/manuscripts/5576/slides.pdf (accessed on 17 November 2019).
- Mangoni, A.A.; Guillou, C.; Vanden Eynde, J.J.; Hulme, C.; Jampilek, J.; Li, W.; Prokai-Tatrai, K.; Rautio, J.; Collina, S.; Tuccinardi, T.; et al. Breakthroughs in medicinal chemistry: New targets and mechanisms, new drugs, new hopes–4. Molecules 2019, 24, 130. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.; Khera, R.A.; Simpson, J. N-(2-Fluorophenyl)cinnamamide. Acta Cryst. E 2010, 66, o533–o534. [Google Scholar] [CrossRef] [Green Version]
- Nissa, M.N.; Aravindan, P.G.; Kasinath, V.; Gopalakrishnan, G.; Merazig, H.; Velmurugan, D. Crystal structures of 2-chloro cinnamoyl phenolate (I) and 3-chloro cinnamanilide (II). Cryst. Res. Technol. 2004, 39, 643–649. [Google Scholar] [CrossRef]
- Skolyapova, A.D.; Selivanova, G.A.; Tretyakov, E.V.; Bogdanova, T.F.; Shchegoleva, L.N.; Bagryanskaya, I.Y.; Gurskaya, L.Y.; Shteingarts, V.D. Interaction of polyfluorinated 2-chloroquinolines with ammonia. Tetrahedron 2017, 73, 1219–1229. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Unger, S.H.; Kim, K.H.; Nikaitani, D.; Lien, E.J. “Aromatic” substituent constants for structure-activity correlations. J. Med. Chem. 1973, 16, 1207–1216. [Google Scholar] [CrossRef]
- Kucerova-Chlupacova, M.; Opletalova, V.; Jampilek, J.; Dolezel, J.; Dohnal, J.; Pour, M.; Kunes, J.; Vorisek, V. New hydrophobicity constants of substituents in pyrazine rings derived from RP-HPLC study. Coll. Czech. Chem. Commun. 2008, 73, 1–18. [Google Scholar] [CrossRef]
- Adewoyin, M.; Mohsin, S.M.N.; Arulselvan, P.; Hussein, M.Z.; Fakurazi, S. Enhanced anti-inflammatory potential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. Drug Des. Dev. Ther. 2015, 9, 2475–2484. [Google Scholar] [CrossRef] [Green Version]
- Jan, J.S.; Chou, Y.C.; Cheng, Y.W.; Chen, C.K.; Huang, W.J.; Hsiao, G. The novel HDAC8 inhibitor WK2-16 attenuates lipopolysaccharide-activated matrix metalloproteinase-9 expression in human monocytic cells and improves hypercytokinemia in vivo. Int. J. Mol. Sci. 2017, 18, 1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-kappa B. Cell. Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappa B signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Tsai, C.M.; Sun, F.M.; Chen, Y.L.; Hsu, C.L.; Yen, G.C.; Weng, C.J. Molecular mechanism depressing PMA-induced invasive behaviors in human lung adenocarcinoma cells by cis- and trans-cinnamic acid. Eur. J. Pharm. Sci. 2013, 48, 494–501. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Cinnamon subcritical water extract attenuates intestinal inflammation and enhances intestinal tight junction in a Caco-2 and RAW264.7 co-culture model. Food Funct. 2019, 10, 4350–4360. [Google Scholar] [CrossRef]
- Lin, F.L.; Yen, J.L.; Kuo, Y.C.; Kang, J.J.; Cheng, Y.W.; Huang, W.J.; Hsiao, G. HADC8 inhibitor WK2-16 therapeutically targets lipopolysaccharide-induced mouse model of neuroinflammation and microglial activation. Int. J. Mol. Sci. 2019, 20, 410. [Google Scholar] [CrossRef] [Green Version]
- D’Acquisto, F.; May, M.J.; Ghosh, S. Inhibition of nuclear factor kappa B (NF-kB): An emerging theme in anti-inflammatory therapies. Mol. Interv. 2002, 2, 22–35. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.Y.; Joo, D.; Sun, S.C. NF-kappa B signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierda, R.J.; Geutskens, S.B.; Jukema, J.W.; Quax, P.H.A.; van den Elsen, P.J. Epigenetics in atherosclerosis and inflammation. J Cell. Mol. Med. 2010, 14, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Zelova, H.; Hosek, J. TNF-alpha signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Jana, M.; Roy, A.; Pahan, K. Upregulation of suppressor of cytokine signaling 3 in microglia by cinnamic acid. Curr. Alzheimer Res. 2018, 15, 894–904. [Google Scholar] [CrossRef]
- Xu, F.; Wang, F.; Wen, T.Q.; Sang, W.T.; He, X.Y.; Li, L.; Zeng, N. Protective effect of cinnamic acid in endotoxin-poisoned mice. Phytother. Res. 2017, 31, 1946–1953. [Google Scholar] [CrossRef]
- Bruker. Apex3; Bruker AXS Inc.: Madison, WI, USA, 2015; Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/apex3.html (accessed on 17 November 2019).
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond Version 4.6.0.; Crystal Impact GbR: Bonn, Germany, 2019. [Google Scholar]
- Plavcova, Z.; Salamunova, P.; Salon, I.; Stepanek, F.; Hanus, J.; Hosek, J. Curcumin encapsulation in yeast glucan particles promotes its anti-inflammatory potential in vitro. Int. J. Pharm. 2019, 568, 118532. [Google Scholar] [CrossRef]
- Vančo, J.; Trávníček, Z.; Hošek, J.; Suchý, P. In vitro and in vivo anti-inflammatory active copper(II)-lawsone complexes. PLoS ONE 2017, 12, e0181822. [Google Scholar] [CrossRef] [Green Version]
- Brezani, V.; Lelakova, V.; Hassan, S.T.S.; Berchova-Bimova, K.; Novy, P.; Kloucek, P.; Marsik, P.; Dall’Acqua, S.; Hosek, J.; Smejkal, K. Anti-Infectivity against herpes simplex virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus Labill. Viruses 2018, 10, E360. [Google Scholar] [CrossRef] [Green Version]
- Leláková, V.; Šmejkal, K.; Jakubczyk, K.; Veselý, O.; Landa, P.; Václavík, J.; Bobál, P.; Pížová, H.; Temml, V.; Steinacher, T.; et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019, 285, 431–440. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








| Formula | C15H11BrFNO |
|---|---|
| Formula weight | 320.16 |
| Temperature | 150(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions | a = 9.410(3) Å, α = 90° |
| b = 14.645(5) Å, β = 108.633(15)° | |
| c = 10.184(4) Å, γ = 90° | |
| Volume | 1329.9(8) Å3 |
| Z | 4 |
| Density (calculated) | 1.599 g/cm3 |
| Absorption coefficient | 3.093 mm−1 |
| F(000) | 640 |
| Crystal size | 0.140 × 0.140 × 0.060 mm |
| Theta range for data collection | 2.284 to 24.999° |
| Index ranges | −11 ≤ h ≤ 11, −17 ≤ k ≤ 17, −12 ≤ l ≤ 12 |
| Reflections collected | 22311 |
| Independent reflections | 2339 [R(int) = 0.1233] |
| Absorption correction | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2339/0/160 |
| Goodness-of-fit on F2 | 1.067 |
| Final R indices [I > 2σ(I)] | R1 = 0.0523, wR2 = 0.1295 |
| R indices (all data) | R1 = 0.0746, wR2 = 0.1391 |
| Largest diff. peak and hole | 1.162 and −1.065 e.Å−3 |
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
|---|---|---|---|---|
| N(1)-H(1)···O(1)(i) | 0.88 | 2.20 | 3.065(5) | 167.5 |
| C(2)-H(2) ···O(1)(i) | 0.95 | 2.71 | 3.507(6) | 141.8 |
| Br(1)···O(1)(i) | – | – | 3.346(3) | – |
 | |||||
|---|---|---|---|---|---|
| Comp. | R | IC50 [µM] ± SEM | NF-κB inhibition [%] | πAr a | MV a [cm3] |
| 1 | H | >30 | 0 b | 1.76 | 80.88 |
| 2 | 3-CH3 | >30 | 9.00 b | 2.22 | 97.11 |
| 3 | 4-CH3 | >30 | 8.50 b | 2.22 | 97.11 |
| 4 | 2-F | >30 | 2.87 b | 2.26 | 85.90 |
| 5 | 3-F | >30 | 9.67 b | 1.78 | 85.90 |
| 6 | 3-CF3 | 11.60 ± 1.13 | 16.67 b | 2.73 | 113.57 |
| 7 | 2,5-CH3 | >30 | 14.78 b | 2.68 | 113.27 |
| 8 | 2,5-Cl | >30 | 22.50b | 2.73 | 103.54 |
| 9 | 2,6-Cl | >30 | 14.11 b | 2.72 | 103.54 |
| 10 | 3,4-Cl | 6.28 ± 2.32 | 10.89 c | 2.77 | 103.54 |
| 11 | 3,5-Cl | 2.43 ± 1.06 | 16.22 c | 2.90 | 103.54 |
| 12 | 2,6-Br | >30 | 23.12b | 2.85 | 107.33 |
| 13 | 3,5-CF3 | 2.17 ± 1.19 | 19.67 c | 3.98 | 145.57 |
| 14 | 2-F-5-Br | >30 | 19.67 b | 2.87 | 99.09 |
| 15 | 2-Br-5-F | >30 | 17.78 b | 2.28 | 99.09 |
| 16 | 2-Cl-4-Br | >30 | 13.67 b | 3.09 | 105.44 |
| 17 | 2-Cl-5-CF3 | >30 | 27.12b | 3.28 | 124.72 |
| 18 | 2-OCH3-5-NO2 | >30 | 17.56 b | 1.12 | 115.77 |
| CA | – | >30 | 11.44 b | – | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hošek, J.; Kos, J.; Strhársky, T.; Černá, L.; Štarha, P.; Vančo, J.; Trávníček, Z.; Devínsky, F.; Jampílek, J. Investigation of Anti-Inflammatory Potential of N-Arylcinnamamide Derivatives. Molecules 2019, 24, 4531. https://doi.org/10.3390/molecules24244531
Hošek J, Kos J, Strhársky T, Černá L, Štarha P, Vančo J, Trávníček Z, Devínsky F, Jampílek J. Investigation of Anti-Inflammatory Potential of N-Arylcinnamamide Derivatives. Molecules. 2019; 24(24):4531. https://doi.org/10.3390/molecules24244531
Chicago/Turabian StyleHošek, Jan, Jiří Kos, Tomáš Strhársky, Lucie Černá, Pavel Štarha, Ján Vančo, Zdeněk Trávníček, Ferdinand Devínsky, and Josef Jampílek. 2019. "Investigation of Anti-Inflammatory Potential of N-Arylcinnamamide Derivatives" Molecules 24, no. 24: 4531. https://doi.org/10.3390/molecules24244531