The Genus Nerine Herb. (Amaryllidaceae): Ethnobotany, Phytochemistry, and Biological Activity
Abstract
:1. Introduction
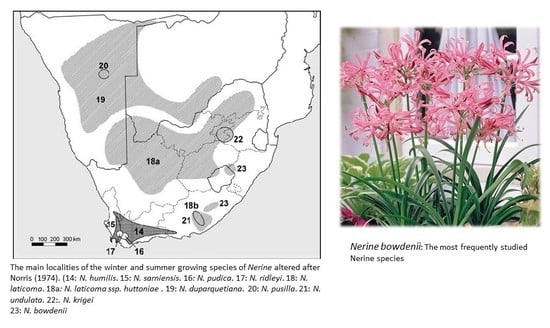
2. The Genus Nerine: Occurrence and Ethnobotany
3. Phytochemistry
4. Biological Activity
4.1. Biological Activity Connected with Potential Treatment of Alzheimer′s Disease
4.2. Antitumor Activity
4.3. Further Biological Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef]
- Hoshino, O. The Amaryllidaceae alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 1998; Volume 51, pp. 323–424. [Google Scholar]
- Dalecká, M.; Havelek, R.; Královec, K.; Brůčková, L.; Cahlíková, L. Amaryllidaceae family alkaloids as potential drugs for cancer treatment. Chem. Listy 2013, 107, 701–708. [Google Scholar]
- He, M.; Qu, C.; Gao, O.; Hu, X.; Hong, X. Biological and pharmacological activities of Amaryllidaceae alkaloids. RSC Adv. 2015, 5, 16562–16574. [Google Scholar] [CrossRef]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2016, 33, 1318–1343. [Google Scholar] [CrossRef]
- Dahlgren, R.M.T.; Clifford, H.T.; Yeo, P.F. The Families of the Monocotyledons. Structure, Evolution and Taxonomy; Springer: New York, NY, USA, 1985. [Google Scholar]
- Bremer, K.; Chase, M.C.; Stevens, P. An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 1998, 85, 531–553. [Google Scholar]
- Zonneveld, B.J.M.; Duncan, G.D. Genome size for the species of Nerine Herb. (Amaryllidaceae) and its evident correlation with growth cycle, leaf width and other morphological characters. Pl. Syst. Evol 2006, 257, 251–260. [Google Scholar] [CrossRef]
- Snijman, D.A.; Linder, H.P. Phylogenetic relationships, seeds characters, and dispersal system evolution in Amaryllideae (Amaryllidaceae). Ann. Mo. Bot. Gard. 1996, 83, 362–386. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa: Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animals; E.& S. Livingston Ltd.: Edinburgh, UK, 1962; pp. 30–32. [Google Scholar]
- Nair, J.J.; Campbell, W.E.; Brun, R.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Nerine filifolia. Phytochemistry 2005, 6, 373–382. [Google Scholar] [CrossRef]
- Chase, M.W.; Reveal, J.L.; Fay, M.F. A substantial classification for the expanded Asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot. J. Linn. Soc. 2009, 161, 132–136. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Kutchan, T.M. The Amaryllidaceae alkaloids: Biosynthesis and methods for enzyme discovery. Phytochem. Rev. 2016, 15, 317–337. [Google Scholar] [CrossRef]
- Takos, A.M.; Rook, F. Towards a molecular understanding of the biosynthesis of Amaryllidaceae alkaloids in support of their expanding medical use. Int. J. Mol. Sci. 2013, 14, 11713–11741. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, R.M.; Rhee, I.K.; Verpoorte, R. Isolation of acetylcholinesterase inhibitory alkaloids from Nerine bowdenii. Nat. Prod. Res. 2010, 24, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Zavadil, S.; Macáková, K.; Valterová, I.; Kulhánková, A.; Hošťálková, A.; Kuneš, J.; Opletal, L. Isolation and cholinesterase activity of Amaryllidaceae alkaloids from Nerine bowdenii. Nat. Prod. Commun. 2011, 6, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Vaněčková, N.; Hošt’álková, A.; Šafratová, M.; Kuneš, J.; Hulcová, D.; Hrabinová, M.; Doskočil, I.; Štěpánková, Š.; Opletal, L.; Nováková, L.; et al. Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W.Watson and their biological activities. RSC Adv. 2016, 6, 80114–80120. [Google Scholar] [CrossRef]
- Molander, M.; Christensen, S.B.; Jäger, A.K.; Olsen, C.E.; Rønsted, N. 6-O-Methylkrigeine, a new Amaryllidaceae alkaloid from Nerine huttoniae Schönland. Nat. Prod. Res. 2012, 26, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cala, A.; Tabanca, N.; Cimmino, A.; Green, I.R.; Bloomquist, J.R.; van Otterlo, W.A.L.; Macias, F.A.; Evidente, A. Alkaloids with activity against Zika virus vector Aedes aegypti (L.)—Crisarnine and Sarniensinol, two new crinine and mesembrine type alkaloids isolated from the South African plant Nerine sarniensis. Molecules 2016, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; van der Westhuyzen, A.E.; Tabanca, N.; Evidente, M.; Cimmino, A.; Green, I.R.; Bernier, U.R.; Becnel, J.J.; Bloomquist, J.R.; van Otterlo, W.A.; et al. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis, an indigenous South African Amaryllidaceae with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia 2017, 116, 34–38. [Google Scholar] [CrossRef]
- Rhee, I.K.; Appels, N.; Hofte, B.; Karabatak, B.; Erkelens, C.; Stark, L.M.; Flippin, L.A.; Verpoorte, R. Isolation of the acetylcholinesterase inhibitor ungeremine from Nerine bowdenii by preparative HPLC coupled on-line to a flow assay system. Biol. Pharm. Bull. 2004, 27, 1804–1809. [Google Scholar] [CrossRef]
- Tanahashi, T.; Poulev, A.; Zenk, M.H. Radioimmunoassay for the quantitative determination of galanthamine. Planta Med. 1990, 56, 77–81. [Google Scholar] [CrossRef]
- Lyle, R.E.; Kielar, E.A.; Crowder, J.R.; Wildman, W.C. Alkaloids of Amaryllidaceae. XV. The alkaloid of Nerine bowdenii W. Wats. and Crinum moorei J.D.Hook. J. Am. Chem. Soc. 1960, 80, 2620–2625. [Google Scholar] [CrossRef]
- Chen, C.K.; Lin, F.H.; Tseng, L.H.; Jiang, C.L.; Lee, S.S. Comprehensive study of alkaloids from Crinum asiaticum varsinicum assisted by HPLC-DAD-SPE-NMR. J. Nat. Prod. 2011, 74, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Benešová, N.; Macáková, K.; Kučera, R.; Hrstka, V.; Klimeš, J.; Jahodář, L.; Opletal, L. Alkaloids from some Amaryllidaceae species and their cholinesterase activity. Nat. Prod. Commun. 2012, 7, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Boit, H.G. Alkaloide von Chlidanthus fragrans, Vallota purpurea, Nerine undulata und Hippeastrum vittatum (XI. Mitteil. Über Amaryllidaceen-Alkaloide). Chem. Ber. 1956, 89, 1129–1134. [Google Scholar] [CrossRef]
- Cahlíková, L.; Benešová, N.; Macáková, K.; Urbanová, K.; Opletal, L. GC/MS analysis of three Amaryllidaceae species and their cholinesterase activity. Nat. Prod. Commun. 2011, 6, 1255–1258. [Google Scholar]
- Pettit, G.R.; Gaddamidi, V.; Goswami, A.; Cragg, G.M. Antineoplastic agents, 99 Amaryllis belladonna. J. Nat. Prod. 1984, 47, 796–801. [Google Scholar] [CrossRef]
- Machocho, A.; Chhabra, S.C.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Ammocharis tinneana. Phytochemistry 1999, 51, 1185–1191. [Google Scholar] [CrossRef]
- Viladomat, F.; Codina, C.; Bastida, J.; Mathee, S.; Campbell, W.E. Further alkaloids from Brunsvigia josephinae. Phytochemistry 1995, 40, 961–965. [Google Scholar] [CrossRef]
- Crain, W.O.; Wildman, W.C.; Roberts, J.D. Nuclear magnetic resonance spectroscopy. Carbon-13 spectra of nicotine, quinine, and some Amaryllidaceae alkaloids. J. Am. Chem. Soc. 1971, 40, 961–965. [Google Scholar]
- Fales, H.M.; Wildman, W.C. Alkaloids of the Amaryllidaceae. XIX. On the structures of crinamidine, flexinine, and nerbowdine. J. Org. Chem. 1961, 26, 181–187. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tokumoto, T.; Kihara, M.; Imakura, Y.; Shingu, T.; Taira, Z. Alkaloidal constituents of Crinum latifolium and Crinum bulbispermum (Amaryllidaceae). Chem. Pharm. Bull. 1984, 32, 3015–3022. [Google Scholar] [CrossRef]
- Jegorov, A.; Buchta, M.; Sedmera, P.; Kuzma, M.; Havlíček, V. Accurate product ion mass spectra of galanthamine derivatives. J. Mass Spectrom. 2006, 41, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.; Lavilla, R.; Viladomat, F. The Alkaloids: Chemical and biological aspects of Narcissus alkaloids. In The Alkaloids: Chemistry and Biology; Chapter 3; Elsevier: Amsterdam, The Netherlands, 2006; pp. 87–179. [Google Scholar] [PubMed]
- Garbutt, D.F.C.; Jeffs, P.W.; Warren, F.L. Alkaloids of Amaryllidaceae. XI. Alkaloids of Nerine krigeii and structure of krigenamine. J. Am. Chem. Soc. 1962, 80, 5010–5014. [Google Scholar] [CrossRef]
- Kreh, M.; Matusch, R. O-Methyloduline and N-Demethylmasonine, alkaloids from Narcissus pseudonarcissus. Phytochemistry 1995, 38, 1533–1535. [Google Scholar] [CrossRef]
- de Andrade, J.P.; Guo, Y.; Font-Bardia, M.; Calvet, T.; Dutilh, J.; Viladomat, F.; Codina, C.; Nair, J.J.; Zuanazzi, J.A.S.; Bastida, J. Crinine-type alkaloids from Hippeastrum aulicum and H. calyptratum. Phytochemistry 2014, 103, 188–195. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Giordani, R.B.; Torras-Claveria, L.; Pigni, N.B.; Berkov, S.; Font-Bardia, M.; Calvet, T.; Konrath, E.; Bueno, K.; Sachett, G.; et al. The Brazilian Amaryllidaceae as a source of acetylcholinesterase inhibitory alkaloids. Phytochem. Rev. 2015, 15, 147–160. [Google Scholar] [CrossRef]
- Wildman, W.C.; Kaufman, C.J. Alkaloids of the Amaryllidaceae. V. Alkaloids of Nerine falcata Barker and N. laticoma (Ker) Dur. and Schinz. J. Am. Chem. Soc. 1955, 77, 4807–4808. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Andolfi, A.; van Goietsenoven, G.; Cimmino, A.; le Calvé, B.; Wauthoz, N.; Mégalizzi, V.; Gras, T.; Bruyère, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, S.; Rao, P.H.; Jaiswal, D.K.; Kumar, Y.; Frahm, A.W. Alkaloids of Crinum pratense. Phytochemistry 1981, 20, 2003–2007. [Google Scholar] [CrossRef]
- Torres, J.C.; Pinto, A.C.; Garden, S.J. Application of a catalytic palladium biaryl synthesis reaction, via C-H functionalization, to the total synthesis of Amaryllidaceae alkaloids. Tetrahedron 2004, 60, 9889–9900. [Google Scholar] [CrossRef]
- Cedrón, J.C.; Gutiérrez, D.; Flores, N.; Ravelo, Á.G.; Estévez-Braun, A. Synthesis and antiplasmodial activity of lycorine derivatives. Bioorgan. Med. Chem. 2010, 18, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Viladomat, F.; Bastida, J.; Tribo, G.; Codina, C.; Rubiralta, M. Alkaloids from Narcissus bicolor. Phytochemistry 1990, 29, 1307–1310. [Google Scholar] [CrossRef]
- Dold, T.; Weeks, D.C. Nerine huttoniae: A rare endemic from the Fish River. Veld and Flora 2000, 86, 14–15. [Google Scholar]
- Meerow, A.W.; Snijman, D.A. Amaryllidaceae. In Flowering plants Monocotyledons; Kubinski, K., Ed.; Springer: Berlin, Germany, 1998; pp. 83–110. [Google Scholar]
- Ding, Y.; Qu, D.; Zhang, K.-M.; Cang, X.-X.; Kou, Z.-N.; Xiao, W.; Zhu, J.-B. Phytochemical and biological investigations of Amaryllidaceae alkaloids: A review. J. Asian Nat. Prod. Res. 2016, 19, 53–100. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.T.N.; Titorenkova, T.V.; Bankova, V.S.; Handjieva, N.V.; Popov, S.S. Crinum L. (Amaryllidaceae). Fitoterapia 2000, 73, 183–208. [Google Scholar] [CrossRef]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15, PCC.12r01412. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [Green Version]
- Giacobini, E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr. Psych. 2003, 18, S1–S5. [Google Scholar] [CrossRef]
- Fulton, B.; Benfield, P. Galanthamin. Drug Aging 1996, 9, 60–65. [Google Scholar] [CrossRef]
- Rhee, I.K.; Appels, N.; Luijendijk, T.; Irth, H.; Verpoorte, R. Determining acetylcholinesterase inhibitory activity in plant extracts using a fluometric flow assay. Phytochem. Anal. 2003, 14, 145–149. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Hostettman, J.; Borloz, A.; Urbain, A.; Marston, A. Natural product inhibitors of acetylcholinesterase. Curr. Org. Chem. 2006, 10, 825–847. [Google Scholar] [CrossRef]
- Houghton, P.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Elgorashi, E.E.; Stafford, G.I.; van Staden, J. Acetylcholinesterase enzyme inhibitory effects of Amaryllidaceae alkaloids. Planta Med. 2004, 70, 260–262. [Google Scholar] [PubMed]
- Cahlíková, L.; Pérez, D.I.; Štěpánková, Š.; Chlebek, J.; Šafratová, M.; Hošťálková, A.; Opletal, L. In vitro inhibitory effects of 8-O-demethylmaritidine and undulatine on acetylcholinesterase and their predicted penetration across the blood−brain barrier. J. Nat. Prod. 2015, 78, 1189–1192. [Google Scholar] [CrossRef]
- Hulcová, D.; Maříková, J.; Korábečný, J.; Hošťálková, A.; Jun, D.; Kuneš, J.; Chlebek, J.; de Simone, A.; Nováková, L.; Andrisano, V.; et al. Amaryllidaceae alkaloids from Narcissus pseudonarcissus L. cv. Dutch Master as potential drugs in treatment of Alzheimer’s disease. Phytochemistry 2019, 165, 112055. [Google Scholar] [CrossRef]
- Babková, K.; Korábečný, J.; Soukup, O.; Nepovímová, E.; Jun, D.; Kuča, K. Prolyl oligopeptidase and its role in the organism: Attention to the most promising and clinically relevant inhibitors. Future. Med. Chem. 2017, 9, 1015–1038. [Google Scholar] [CrossRef]
- Goossens, F.; De Meester, I.; Vanhoof, G.; Scharpé, S. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 17–22. [Google Scholar] [CrossRef] [Green Version]
- García-Horsman, J.A.; Männistö, P.T.; Venäläinen, J.I. On the role of prolyl oligopeptidase in health and disease. Neuropeptides 2007, 41, 1–24. [Google Scholar] [CrossRef]
- Šafratová, M.; Novák, Z.; Kulhánková, A.; Kuneš, J.; Hrabinová, M.; Jun, D.; Macáková, K.; Opletal, L.; Cahlíková, L. Revised NMR data for 9-O-demethylgalanthine: An alkaloid from Zephyranthes robusta (Amaryllidaceae) and its biological activity. Nat. Prod. Commun. 2014, 9, 787–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evidente, A.; Kireev, A.S.; Jenkins, A.R.; Romero, A.E.; Steelant, W.F.; Van Slambrouck, S.; Kornienko, A. Biological evaluation of structurally diverse amaryllidaceae alkaloids and their synthetic derivatives: Discovery of novel leads for anticancer drug design. Planta Med. 2009, 75, 501–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Goietsenoven, G.V.; Andolfi, A.; Lallemand, B.; Cimmino, A.; Lamoral-Theys, D.; Gras, T.; Abou-Donia, A.; Dubois, J.; Lefranc, F.; Mathieu, V.; et al. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells. J. Nat. Prod. 2010, 73, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Doskočil, I.; Hošťálková, A.; Šafratová, M.; Benešová, N.; Havlík, J.; Havelek, R.; Kuneš, J.; Královec, K.; Chlebek, J.; Cahlíková, L. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochem. Lett. 2015, 13, 394–398. [Google Scholar] [CrossRef]
- Nair, J.J.; van Staden, J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat. Prod. Commun. 2014, 9, 1193–1210. [Google Scholar] [CrossRef]
- Hu, M.; Peng, S.; He, Y.; Qin, M.; Cong, X.; Xing, Y.; Liu, M.; Yi, Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget. 2015, 6, 15348–15361. [Google Scholar] [CrossRef] [Green Version]
- Habartová, K.; Cahlíková, L.; Řezáčová, M.; Havelek, R. From cholinesterases inhibition to anticancer activity. Nat. Prod. Commun. 2016, 11, 1587–1594. [Google Scholar]
- Antoun, M.D.; Mendoza, N.T.; Ríos, Y.R.; Proctor, G.R.; Wickramaratne, D.B.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxicity of Hymenocallis expansa alkaloids. J. Nat. Prod. 1993, 56, 1423–1425. [Google Scholar] [CrossRef]
- Nair, J.J.; Rárová, L.; Strnad, M.; Bastida, J.; van Staden, J. Apoptosis-inducing effects of distichamine and narciprimine, rare alkaloids of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2012, 22, 6195–6199. [Google Scholar] [CrossRef]
- Nair, J.J.; Bastida, J.; Viladomat, F.; van Staden, J. Cytotoxic agents of the crinane series of amaryllidaceae alkaloids. Nat. Prod. Commun. 2012, 7, 1677–6188. [Google Scholar] [CrossRef]
- Furusawa, E.; Irie, H.; Combs, D.; Wildman, W.C. Therapeutic activity of pretazettine on Rauscher leukemia: Comparison with the related Amaryllidaceae alkaloids. Chemotherapy 1980, 26, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Havelek, R.; Muthna, D.; Tomsik, P.; Kralovec, K.; Seifrtova, M.; Cahlikova, L.; Hostalkova, A.; Safratova, M.; Perwein, M.; Cermakova, E.; et al. Anticancer potential of Amaryllidaceae alkaloids evaluated by screening with a panel of human cells, real-time cellular analysis and Ehrlich tumor-bearing mice. Chem. Biol. Interact. 2017, 275, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Havelek, R.; Seifrtova, M.; Kralovec, K.; Bruckova, L.; Cahlikova, L.; Dalecka, M.; Vavrova, J.; Rezacova, M.; Opletal, L.; Bilkova, Z. The effect of Amaryllidaceae alkaloids haemanthamine and haemanthidine on cell cycle progression and apoptosis in p53-negative human leukemic Jurkat cells. Phytomedicine 2014, 21, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Hroch, M.; Mičuda, S.; Havelek, R.; Cermanová, J.; Cahlíková, L.; Hošťálková, A.; Hulcová, D.; Řezáčová, M. LC-MS/MS method for determination of haemanthamine in rat plasma, bile and urine: Application to a pilot pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.C. Zika highlights role of fetal-tissue research. Nature 2016, 532, 16. [Google Scholar] [CrossRef] [Green Version]
- WHO. Dengue vaccine: WHO position paper, July 2016—Recommendations. Vaccine 2017, 35, 1200–1201. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| N. Bowdenii | N. Corusca | N. Falcata | N. Filamentosa | N. Filifolia | N. Flexuosa | N. Huttoniae | N. Krigeii | N. Laticoma | N. Sarniensis | N. Undulata | Ref for NMR, MS Data | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belladine-type | ||||||||||||
| Belladine (1) | [16,17,23] | [11] | [11,16] | |||||||||
| 4′-O-Demethylbelladine (2) | [17] | [17] | ||||||||||
| 6-O-Demethylbelladine (3) | [17] | [17] | ||||||||||
| N-Demethylbelladine (4) | [16] | [11] | [11,16] | |||||||||
| Crinine-type | ||||||||||||
| 1-O-Acetylbulbisine (5) | [17] | [24] | ||||||||||
| Acetylnerbowdine (6) | [16] | [16] | ||||||||||
| Ambelline (7) | [15,16,17,23] | [25] | [11] | [26,27] | [25,28] | |||||||
| 11-O-Acetyl-1,2-epoxy- ambelline (8) | [16] | [16,29] | ||||||||||
| 11-O-Acetylambelline (9) | [15,16,17] | [11] | [16,30] | |||||||||
| 1,2-Epoxyambelline (10) | [17] | [17,29] | ||||||||||
| Bowdensine (11) | [16,23] | [19] | [27] | [16,19] | ||||||||
| Buphanamine (12) | [16,17] | [25] | [27] | [16,31] | ||||||||
| 6α-Hydroxy-buphanidrine (13) | [11] | [11] | ||||||||||
| 6α-Methoxy-buphanidrine (14) | [11] | [11] | ||||||||||
| Buphanidrine (15) | [16,17,23] | [27] | [16,30] | |||||||||
| Buphanisine (16) | [15,16,17,23] | [16,30] | ||||||||||
| Crinamidine (17) | [15,16,23,32] | [33] | [32] | [27] | [16,33] | |||||||
| Crinamine (18) | [16,23] | [16,33] | ||||||||||
| Crinine (19) | [15,16,23] | [25] | [16,30,33] | |||||||||
| Crinsarnine (20) | [19] | [19] | ||||||||||
| Deacetylbowdesine (21) | [17] | [17] | ||||||||||
| Filifoline (22) | [15] | [11] | [11] | |||||||||
| Flexinine (23) | [27] | [27,29] | ||||||||||
| Nerbowdine (24) | [23] | [23] | ||||||||||
| Powelline (25) | [15,16] | [16,33] | ||||||||||
| Undulatine (26) | [15,16,17,23] | [25] | [11] | [26,27] | [16,28,29,30] | |||||||
| 6-Hydroxyundulatine (27) | [17] | [29] | ||||||||||
| Galanthamine-type | ||||||||||||
| Galanthamine (28) | [22] | [25,34] | ||||||||||
| Haemanthamine-type | ||||||||||||
| Haemanthamine (29) | [17] | [27] | [25,35] | |||||||||
| Hammayne (30) | [17] | [33] | ||||||||||
| Homolycorine-type | ||||||||||||
| Krigeine (31) | [36] | [36] | ||||||||||
| 6-O-Methylkrigeine (32) | [18] | [18] | ||||||||||
| Krigenamine (33) | [36] | [36] | ||||||||||
| Oxokrigenamine (34) | [18] | [18] | ||||||||||
| Masonine (35) | [25] | [25,37] | ||||||||||
| N-Demethylmasonine (36) | [25] | [25,37] | ||||||||||
| Nerinine (37) | [36] | [38,39] | ||||||||||
| O-Methyloduline (38) | [25] | [25,37] | ||||||||||
| Lycorine-type | ||||||||||||
| Caranine (39) | [15,16] | [40] | [25] | [40] | [16,41] | |||||||
| Acetylcaranine (40) | [15,16] | [25] | [16,28] | |||||||||
| Falcatine (41) | [40] | [27] | [27,40] | |||||||||
| Acetylfalcatine (42) | [16] | [16] | ||||||||||
| Hippadine (43) | [19] | [42,43] | ||||||||||
| Lycorine (44) | [23] | [40] | [25] | [25] | [18] | [36] | [40] | [26] | [18,25,41] | |||
| 1-O-Acetyllycorine (45) | [15,23] | [19] | [41,44] | |||||||||
| Parkamine (46) | [25] | [25] | ||||||||||
| Acetylparkamine (47) | [25] | [25] | ||||||||||
| Ungeremine (48) | [21] | [21,41] | ||||||||||
| Montanine-type | ||||||||||||
| Montanine (49) | [27] | [27,31] | ||||||||||
| Mesembrine-type | ||||||||||||
| Sarniensine (50) | [20] | [20] | ||||||||||
| Sarniensinol (51) | [19] | [19] | ||||||||||
| Tazettine-type | ||||||||||||
| 3-Epimacronine (52) | [19] | [27] | [27,45] | |||||||||
| Tazettine (53) | [15] | [18] | [19] | [27] | [18,31] |
| Plant Species | AChE, IC50 (µg/mL) | BuChE, IC50 (µg/mL) | POP, IC50 (µg/mL) | Reference |
|---|---|---|---|---|
| N. bowdenii | 87.9 ± 3.571% inhibition of AChE | 14.8 ± 1.1 | n.t. | [27,55] |
| N. filamentosa | 21.6 ± 1.1 | 13.0 ± 0.7 | n.t. | [25] |
| N. filifolia | 18.5 ± 0.5 | 58.6 ± 1.3 | n.t. | [25] |
| N. undulata | 14.3 ± 1.2 | 33.9 ± 1.9 | n.t. | [16] |
| AChE, IC50 (µM) | BuChE, IC50 (µM) | POP, IC50 (µM) | ||
| Undulatine (26) | 23.5 ± 1.2 | >1000 | >1000 | [17] |
| Poweline (25) | 29.1 ± 1.6 | 394.2 ± 4.8 | 770 ± 20 | [17] |
| Ungeremine (48) | 0.35 | n.t | n.t | [21] |
| 6-O-Demethylbelladine (3) | 223.2 ± 23.6 | 115.7 ± 10.1 | 660 ± 90 | [17] |
| 4′-O-Demethylbelladine (2) | 606.8 ± 74.2 | 30.7 ± 4.0 | 370 ± 30 | [17] |
| 1-O-Acetyllycorine (45) | > 1000 | 176.2 ± 14.2 | 450 ± 50 | [17] |
| Crinamidine (17) | 230.1 ± 9.8 | > 1000 | 790 ± 60 | [17] |
| Galanthamine* | 1.7 ± 0.1 | 42.3 ± 1.3 | > 1000 | [17] |
| Berberine* | n.t. | n.t. | 140 ± 2 | [17] |
| Cancer Cells | Normal Cells | ||
|---|---|---|---|
| Compound | Caco-2 IC50 (µM) | HT-29 IC50 (µM) | FHs 74 Int IC50 (µM) |
| Ambelline (7) | 74.1 ± 1.1 | 50.2 ± 1.2 | 89.8 ± 6.5 |
| 11-O-Acetylambelline (9) | > 100 | > 100 | > 100 |
| 1-O-Acetylbulbisine (5) | 33.4 ± 2.9 | 47.9 ± 1.6 | 61.3 ± 8.8 |
| Acetylcaranine (40) | 29.5 ± 0.6 | 19.2 ± 1.2 | 66.1 ± 6.8 |
| Buphanamine (12) | 53.5 ± 0.7 | 47.6 ± 2.2 | > 100 |
| Buphanisine (16) | 8.6 ± 0.2 | 5.3 ± 1.7 | 22.8 ± 2.6 |
| Caranine (39) | 64.4 ± 4.5 | 46.6 ± 1.9 | > 100 |
| Crinine (19) | 64.5± 17.8 | 50.8 ± 1.4 | > 100 |
| Haemanthamine (29) | 1.0 ± 0.1 | 0.6 ± 0.0 | 19.5 ± 8.9 |
| Hamayne (30) | 17.2 ± 0.9 | 12.4 ± 0.3 | 53.3 ± 7.4 |
| Tazettine (53) | 22.8 ± 3.3 | 23.4 ± 2.0 | 71.1 ± 5.2 |
| Undulatine (26) | 51.7 ± 1.1 | 53.4 ± 2.2 | 70.4 ± 6.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahlíková, L.; Vaněčková, N.; Šafratová, M.; Breiterová, K.; Blunden, G.; Hulcová, D.; Opletal, L. The Genus Nerine Herb. (Amaryllidaceae): Ethnobotany, Phytochemistry, and Biological Activity. Molecules 2019, 24, 4238. https://doi.org/10.3390/molecules24234238
Cahlíková L, Vaněčková N, Šafratová M, Breiterová K, Blunden G, Hulcová D, Opletal L. The Genus Nerine Herb. (Amaryllidaceae): Ethnobotany, Phytochemistry, and Biological Activity. Molecules. 2019; 24(23):4238. https://doi.org/10.3390/molecules24234238
Chicago/Turabian StyleCahlíková, Lucie, Nina Vaněčková, Marcela Šafratová, Kateřina Breiterová, Gerald Blunden, Daniela Hulcová, and Lubomír Opletal. 2019. "The Genus Nerine Herb. (Amaryllidaceae): Ethnobotany, Phytochemistry, and Biological Activity" Molecules 24, no. 23: 4238. https://doi.org/10.3390/molecules24234238