Graphene Oxide/Polyethylene Glycol-Stick for Thin Film Microextraction of β-Blockers from Human Oral Fluid by Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Experimental
2.1. Material and Instrumentation
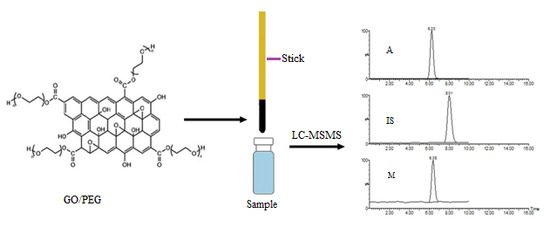
2.2. Synthesis of GO/PEG Hybrid Material
2.3. LC-MS/MS Conditions
2.4. Stock Solutions and Sample Preparation Process
3. Result and Discussions
3.1. Structure of GO/PEG-20000 Hybrid Material
3.2. Method Development
3.2.1. Effect of Adsorption Time
3.2.2. Effect of Desorption Time
3.2.3. Effect of Sample Solution pH
3.2.4. Effect of Desorption Solution
3.2.5. Effect of Sample Ionic Strength
3.2.6. GO/PEG-Stick Extraction Capacity
3.2.7. Selectivity of GO/PEG-Stick
3.2.8. Reusability of the GO/PEG-Stick
3.3. Method Validation
3.3.1. Calibration, Accuracy, and Precision
3.3.2. Limit of Detection and Quantification
3.3.3. Carry-Over
3.3.4. Matrix Effect
3.4. Method Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 May 2017).
- Cruickshank, J.M.; Prichard, B.N.C. Beta-Blockers in Clinical Practice; Churchill Livingstone Inc.: New York, NY, USA, 1988. [Google Scholar]
- Porterfield, J. Doping: Athletes and Drugs; The Rosen Publishing Group: New York, NY, USA, 2007. [Google Scholar]
- Akbay, C.; Rizvi, S.A.A.; Shamsi, S.A. Simultaneous enantioseparation and tandem UV-MS detection of eight b-blockers in micellar electrokinetic chromatography using a chiral molecular micelle. Anal. Chem. 2005, 77, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Paik, M.J.; Lee, J.; Kim, K.R. Simultaneous screening analysis of multiple β-blockers in urine by gas chromatography–mass spectrometry in selected ion monitoring mode. Anal. Chim. Acta. 2007, 601, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Delamoye, M.; Duverneuil, C.; Paraire, F.; de Mazancourt, P.; Alvarez, J.C. Simultaneous determination of thirteen β-blockers and one metabolite by gradient high-performance liquid chromatography with photodiode-array UV detection. Forensic. Sci. Int. 2004, 141, 23–31. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Liu, X.; Fu, R.; Yang, L. Rapid and sensitive tapered-capillary microextraction combined to on-line sample stacking-capillary electrophoresis for extraction and quantification of two beta-blockers in human urine. Talanta 2018, 180, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Liu, Y.; Yang, C.; Liu, L. On-line double focusing of atenolol and metoprolol in human urine using capillary electrophoresis with the aid of β-cyclodextrin. Anal. Chim. Acta 2017, 978, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.W.; Wu, H.L.; Yin, X.L.; Li, Y.; Liu, Y.J.; Xia, H.; Yang, P.Y. Multi-targeted interference-free determination of ten β-blockers in human urine and plasma samples by alternating trilinear decomposition algorithm-assisted liquid chromatography–mass spectrometry in full scan mode: Comparison with multiple reaction monitoring. Anal. Chim. Acta 2014, 848, 10–24. [Google Scholar] [PubMed]
- Kolmonen, M.; Leinonen, A.; Pelander, A.; Ojanperä, I. A general screening method for doping agents in human urine by solid phase extraction and liquid chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta 2007, 585, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Goryński, K.; Kiedrowicz, A.; Bojko, B. Development of SPME-LC–MS method for screening of eight beta-blockers and bronchodilators in plasma and urine samples. J. Pharm. Biomed. Anal. 2016, 127, 147–155. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Microextraction by packed sorbent (MEPS): A tutorial. Anal. Chim. Acta 2011, 701, 119–128. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Theodoridis, G.; Koster, E.D.; De Jong, G.J. Solid-phase microextraction for the analysis of biological samples. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 49–82. [Google Scholar] [CrossRef]
- Bojko, B. Solid-phase microextraction in metabolomics. TrAC Trends Anal. Chem. 2014, 61, 168–180. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Gionfriddo, E.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices II. Food analysis. TrAC Trends Anal. Chem. 2015, 71, 236–248. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-film microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Zang, X.; Li, J.; Ma, J. Graphene-based solid-phase extraction combined with flame atomic absorption spectrometry for a sensitive determination of trace amounts of lead in environmental water and vegetable samples. Anal. Chim. Acta. 2012, 716, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Leilei, L.; Chuannan, L.; Xiangjun, L.; Huimin, D.; Xiaojiao, W. Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment. Int. J. Biol. Macromol. 2014, 66, 172–178. [Google Scholar]
- Li, C.; Xiang, M.; Ye, L. Intercalation behavior and orientation structure of graphene oxide/polyethylene glycol hybrid material. RSC Adv. 2016, 6, 72193–72200. [Google Scholar] [CrossRef]
- Mehta, S.K.; Umar, A. Highly sensitive hydrazine chemical sensor based on mono-dispersed rapidly synthesized PEG-coated ZnS nanoparticles. Talanta 2011, 85, 2411–2416. [Google Scholar] [CrossRef]
- Liu, X.Y.; Nothias, J.M.; Scavone, A.; Garfinkel, M.; Millis, J.M. Biocompatibility investigation of polyethylene glycol and alginate-poly-l-lysine for islet encapsulation. ASAIO J. 2010, 56, 241–245. [Google Scholar] [CrossRef]
- Abuzooda, T.; Amini, A.; Abdel-Rehim, M. Graphite-based microextraction by packed sorbent for online extraction of β-blockers from human plasma samples. J. Chromatogr. B. 2015, 992, 86–90. [Google Scholar] [CrossRef] [PubMed]
- PubChem Database. Acebutolol, CID=1978 and Metoprolol, CID=4171. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 6 October 2019).
- Wang, Z.; Zhang, X.; Jiang, S.; Guo, X. Magnetic solid-phase extraction based on magnetic multiwalled carbon nanotubes for the simultaneous enantiomeric analysis of five β-blockers in the environmental samples by chiral liquid chromatography coupled with tandem mass spectrometry. Talanta 2018, 180, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, Y.; Fang, J.; Wang, D. Immune response is required for the control of in vivo translocation and chronic toxicity of graphene oxide. Nanoscale 2014, 6, 5894–5906. [Google Scholar] [CrossRef] [PubMed]
- Ashri, N.Y.; Abdel-Rehim, M. Sample treatment based on extraction techniques in biological matrices. Bioanalysis 2011, 3, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Niessen, W.M.A.; Manini, P.; Andreoli, R. Matrix effects in quantitative pesticide analysis using liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2006, 25, 881–899. [Google Scholar] [CrossRef] [PubMed]
- El-Beqqali, A.; Kussak, A.; Blomberg, L.; Abdel-Rehim, M. Microextraction in packed syringe/liquid chromatography/electrospray tandem mass spectrometry for quantification of acebutolol and metoprolol in human plasma and urine samples. J. Liq. Chromatogr. Related Technol. 2007, 30, 575–586. [Google Scholar] [CrossRef]
- Salem, A.A.; Wasfi, I.; Al-Nassib, S.S.; Mohsin, M.A.; Al-Katheeri, N. Determination of Some β-Blockers and β2-Agonists in Plasma and Urine Using Liquid Chromatography–tandem. J. Chromatogr. Sci. 2017, 55, 846–856. [Google Scholar] [CrossRef]
- Kataoka, H.; Narimatsu, S.; Lord, H.L.; Pawliszyn, J. Automated in-tube solid-phase microextraction coupled with liquid chromatography/electrospray ionization mass spectrometry for the determination of beta-blockers and metabolites in urine and serum samples. Anal. Chem. 1999, 71, 4237–4244. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds acebutolol, and metoprolol are available from the authors. |







| Compound | Retention (mins ± SD) | Precursor Ion (m/z) | Product Ion (m/z) | Cone Energy (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| Acebutolol | 6.25 ± 0.015 | 337.15 | 116.1, 141.14, | 25 | 22 |
| Metoprolol | 6.38 ± 0.017 | 268.16 | 116.34, 177,33 | 30 | 20 |
| Pentycaine (I.S) | 8.01 ± 0.012 | 303.37 | 150.17, 154.3 | 35 | 20 |
| Compounds | Samples | Accuracy (%) (n = 12) | Precision (RSD%) | |
|---|---|---|---|---|
| Intra-Day (n = 6) | Intra-Day (n = 12) | |||
| Acebutolol | QCL | 97.0 | 12.3 | 12.9 |
| QCM | 85.0 | 3.15 | 3.6 | |
| QCH | 78.0 | 2.0 | 8.7 | |
| Metoprolol | QCL | 95.0 | 10.5 | 11.3 |
| QCM | 101.0 | 4.7 | 9.5 | |
| QCH | 109.0 | 10.7 | 10.7 | |
| Method | Ex. Sorbent | Sample Volume | Ex. Yield (%) | Accuracy (%) | Matrix | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| mL | ACE | MET | ACE | MET | ||||
| LC-MS/MS | MEPS Polystyrene | 0.1 | 50 | 50 | 98–104 | 94–100 | Plasma | [30] |
| 103–107 | 91–99 | Urine | ||||||
| LC-MS/MS | SPE/ion exchange | 2.0 | 84–96 | - | 84–96 | - | Plasma | [31] |
| >100 | 109–124 | - | Urine | |||||
| LC-MS | In-tube SPME | 1.0 | 5.0 | 2.4 | 82–89 | 72–76 | Serum | [32] |
| 88–94 | 84–85 | Urine | ||||||
| LC-MS/MS | Stick/GO/PEG | 1.0 | 80–90 | 62 | 80–97 | 95–109 | Oral fluid | Present work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimiyan, H.; Hadjmohammadi, M.R.; Kunjali, K.L.; Moein, M.M.; Dutta, J.; Abdel-Rehim, M. Graphene Oxide/Polyethylene Glycol-Stick for Thin Film Microextraction of β-Blockers from Human Oral Fluid by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2019, 24, 3664. https://doi.org/10.3390/molecules24203664
Karimiyan H, Hadjmohammadi MR, Kunjali KL, Moein MM, Dutta J, Abdel-Rehim M. Graphene Oxide/Polyethylene Glycol-Stick for Thin Film Microextraction of β-Blockers from Human Oral Fluid by Liquid Chromatography-Tandem Mass Spectrometry. Molecules. 2019; 24(20):3664. https://doi.org/10.3390/molecules24203664
Chicago/Turabian StyleKarimiyan, Hanieh, Mohammad Reza Hadjmohammadi, Karthik Laxman Kunjali, Mohammad Mahdi Moein, Joydeep Dutta, and Mohamed Abdel-Rehim. 2019. "Graphene Oxide/Polyethylene Glycol-Stick for Thin Film Microextraction of β-Blockers from Human Oral Fluid by Liquid Chromatography-Tandem Mass Spectrometry" Molecules 24, no. 20: 3664. https://doi.org/10.3390/molecules24203664