Optimization of Ultrasonic-Assisted Extraction and Purification of Zeaxanthin and Lutein in Corn Gluten Meal
Abstract
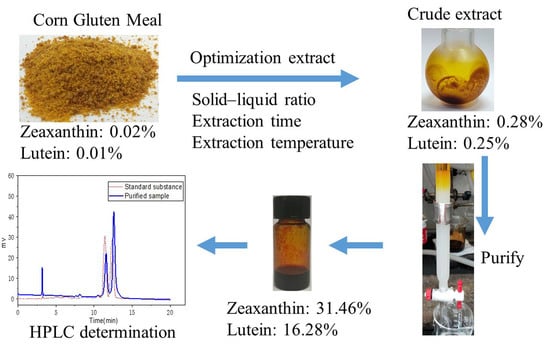
:1. Introduction
2. Results and Discussion
2.1. Zeaxanthin and Lutein Method Development
2.1.1. Precision and Stability Experiments
2.1.2. Reproducibility and Recovery of Zeaxanthin and Lutein
2.1.3. Determination of Sample Content
2.2. Analysis of Single Factor Test Results
2.2.1. Effect of Liquid-to-Solid Ratio on Extraction
2.2.2. Effect of Temperature on Extraction
2.2.3. Effect of Time on Extraction
2.3. Optimization of Extraction by RSM
2.4. Verification of Process Amplification
2.5. Purification of Zeaxanthin and Lutein
3. Materials and Methods
3.1. Samples and Chemicals Reagents
3.2. Sample Preparation
3.3. Zeaxanthin and Lutein Extraction Protocol
3.4. HPLC Identification and Quantification
3.5. Linear Range and Standard Curve Determination
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | high performance liquid chromatography |
| TLC | thin-layer chromatography |
| RSD | relative standard deviation |
References
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohyd. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Xu, Y.; Xu, Y.; Li, H.; Zhao, F.; Wang, C.; Liu, Z.; Liu, P.; Liu, Y.; et al. Synthesis and in vitro anti-inflammatory activity of C20 epimeric ocotillol-type triterpenes and protopanaxadiol. Planta Med. 2019, 85, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Yang, J.; Wang, W.; Zhang, J.; Zhang, R.; Meng, Q. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Guo, Q.; Li, Y.; Jiang, J.; Shi, J. Aromatic compounds from an aqueous extract of “ban lan gen” and their antiviral activities. Acta Pharm. Sin. B 2017, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Rognon, G.T.; Mattinson, T.; Vachali, P.P.; Gorusupudi, A.; Chang, F.Y.; Ranganathan, A.; Nelson, K.; George, E.W.; Frederick, J.M.; et al. Supplementation with macular carotenoids improves visual performance of transgenic mice. Arch. Biochem. Biophys. 2018, 649, 22–28. [Google Scholar] [CrossRef]
- Mewborn, C.M.; Terry, D.P.; Renzi-Hammond, L.M.; Hammond, B.R.; Miller, L.S. Relation of retinal and serum lutein and zeaxanthin to white matter integrity in older adults: A diffusion tensor imaging study. Arch. Clin. Neuropsychol. 2018, 33, 861–874. [Google Scholar] [CrossRef]
- Panova, I.G.; Yakovleva, M.A.; Tatikolov, A.S.; Kononikhin, A.S.; Feldman, T.B.; Poltavtseva, R.A.; Nikolaev, E.N.; Sukhikh, G.T.; Ostrovsky, M.A. Lutein and its oxidized forms in eye structures throughout prenatal human development. Exp. Eye Res. 2017, 160, 31–37. [Google Scholar] [CrossRef]
- Sahin, K.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, N.; Ozercan, I.H.; Ali, S.; Yilmaz, I.; Juturu, V. (3R, 3′R)-zeaxanthin protects the retina from photo-oxidative damage via modulating the inflammation and visual health molecular markers. Cutan. Ocul. Toxicol. 2019, 38, 161–168. [Google Scholar] [CrossRef]
- Hu, J.X.; Lu, W.H.; Lv, M.; Wang, Y.L.; Ding, R.F.; Wang, L.T. Extraction and purification of astaxanthin from shrimp shells and the effects of different treatments on its content. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Juin, C.; de Oliveira, R.G.; Fleury, A.; Oudinet, C.; Pytowski, L.; Berard, J.B.; Nicolau, E.; Thiery, V.; Lanneluc, I.; Beaugeard, L.; et al. Zeaxanthin from porphyridium purpureum induces apoptosis in human melanoma cells expressing the oncogenic BRAF V600E mutation and sensitizes them to the BRAF inhibitor vemurafenib. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2018, 28, 457–467. [Google Scholar] [CrossRef]
- Aguilar, F.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.M.; Grilli, S.; Gürtler, R.; König, J.; Lambré, C.; Larsen, J.-C.; et al. Scientific opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 1678. [Google Scholar]
- Cobb, B.F.; Kallenbach, J.; Hall, C.A.; Pryor, S.W. Optimizing the supercritical fluid extraction of lutein from corn gluten meal. Food Bioprocess Technol. 2018, 11, 757–764. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, S.; Moon, B. Optimization by response surface methodology of lutein recovery from paprika leaves using accelerated solvent extraction. Food Chem. 2016, 205, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Zhang, Y.; Zheng, B.; Li, Q.W.; Zou, Y.H. Microwave-assisted hydrolysis of lutein and zeaxanthin esters in marigold (Tagetes erecta L.). Int. J. Food Sci. Nutr. 2011, 62, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Ho, Y.C.; Lu, F.J. Determination of lutein and zeaxanthin and antioxidant capacity of supercritical carbon dioxide extract from daylily (Hemerocallis disticha). Food Chem. 2011, 129, 1813–1818. [Google Scholar] [CrossRef]
- Yingngam, B.; Zhao, H.; Baolin, B.; Pongprom, N.; Brantner, A. Optimization of ultrasonic-assisted extraction and purification of rhein from cassia fistula pod pulp. Molecules 2019, 24, 2013. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, W.; Jiang, Y.; Wang, H.; Chen, G.; Guo, M. Physicochemical, structural, and biological properties of polysaccharides from dandelion. Molecules 2019, 24, 1485. [Google Scholar] [CrossRef]
- Chu, M.-J.; Du, Y.-M.; Liu, X.-M.; Yan, N.; Wang, F.-Z.; Zhang, Z.-F. Extraction of proanthocyanidins from chinese wild rice (zizania latifolia) and analyses of structural composition and potential bioactivities of different fractions. Molecules 2019, 24, 1681. [Google Scholar] [CrossRef]
- Tan, Z.; Li, Q.; Wang, C.; Zhou, W.; Yang, Y.; Wang, H.; Yi, Y.; Li, F. Ultrasonic assisted extraction of paclitaxel from taxus x media using ionic liquids as adjuvants: optimization of the process by response surface methodology. Molecules 2017, 22, 1483. [Google Scholar] [CrossRef]
- Liu, J.-L.; Zheng, S.-L.; Fan, Q.-J.; Yuan, J.-C.; Yang, S.-M.; Kong, F.-L. Optimization of high-pressure ultrasonic-assisted simultaneous extraction of six major constituents from ligusticum chuanxiong rhizome using response surface methodology. Molecules 2014, 19, 1887–1911. [Google Scholar] [CrossRef]
- Pu, J.-B.; Xia, B.-H.; Hu, Y.-J.; Zhang, H.-J.; Chen, J.; Zhou, J.; Liang, W.-Q.; Xu, P. Multi-optimization of ultrasonic-assisted enzymatic extraction of atratylodes macrocephala polysaccharides and antioxidants using response surface methodology and desirability function approach. Molecules 2015, 20, 22220–22235. [Google Scholar] [CrossRef]
- Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response Surface methodology optimization of ultrasonic-assisted extraction of acer truncatum leaves for maximal phenolic yield and antioxidant activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef]
- Aman, R.; Biehl, J.; Carle, R.; Conrad, J.; Beifuss, U.; Schieber, A. Application of HPLC coupled with DAD, APcI-MS and NMR to the analysis of lutein and zeaxanthin stereoisomers in thermally processed vegetables. Food Chem. 2005, 92, 753–763. [Google Scholar] [CrossRef]
- Warfield, J.R.; Lewis, B.J. Validation of an HPLC Method for the simultaneous determination of trans-lutein, (3R,3′R)-trans-zeaxanthin, and (3R,3′S)-trans-zeaxanthin. J. AOAC Int. 2013, 96, 630–634. [Google Scholar] [CrossRef]
- Prado-Cabrero, A.; Beatty, S.; Stack, J.; Howard, A.; Nolan, J.M. Quantification of zeaxanthin stereoisomers and lutein in trout flesh using chiral high-performance liquid chromatography-diode array detection. J. Food Compos. Anal. 2016, 50, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhang, X.; Cui, Y.; Liu, G.; Li, X.; Chen, S.; Li, Y. Determination of lutein and zeaxanthin in Tagetes erecta L. by HPLC. Chin. J. New Drugs 2006, 15, 1381–1382. [Google Scholar]
- Liu, Y.; Liu, J.; Chen, X.; Liu, Y.; Di, D. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 2010, 123, 1027–1034. [Google Scholar] [CrossRef]
- Pei, D.; Wu, X.; Liu, Y.; Huo, T.; Di, D.; Guo, M.; Zhao, L.; Wang, B. Different ionic liquid modified hypercrosslinked polystyrene resin for purification of catechins from aqueous solution. Colloid. Surf. A 2016, 509, 158–165. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |






| Sample | Precision | Stability | Reproducibility |
|---|---|---|---|
| Zeaxanthin RSD (%) | 1.44 | 1.93 | 0.91 |
| Lutein RSD (%) | 1.77 | 1.73 | 0.75 |
| Run | Factor A: Liquid-Solid Ratio (mL/g) | Factor B: Extraction Temperature (°C) | Factor C: Extraction Time (min) | Y: Extraction Yield (µg/g) |
|---|---|---|---|---|
| 1 | 9 | 50 | 40 | 176.5 |
| 2 | 7 | 60 | 40 | 212.8 |
| 3 | 7 | 70 | 50 | 195.6 |
| 4 | 7 | 60 | 40 | 213.3 |
| 5 | 5 | 60 | 50 | 174.4 |
| 6 | 5 | 60 | 30 | 146.2 |
| 7 | 7 | 60 | 40 | 211.8 |
| 8 | 7 | 50 | 50 | 208.3 |
| 9 | 5 | 50 | 40 | 149.3 |
| 10 | 7 | 70 | 30 | 208.2 |
| 11 | 9 | 70 | 40 | 200.3 |
| 12 | 5 | 70 | 40 | 161.2 |
| 13 | 9 | 60 | 50 | 198.8 |
| 14 | 7 | 50 | 30 | 155.3 |
| 15 | 7 | 60 | 40 | 212.9 |
| 16 | 7 | 60 | 40 | 213.2 |
| 17 | 9 | 60 | 30 | 188.5 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9512.86 | 9 | 1056.98 | 1673.01 | <0.0001 |
| A | 2211.12 | 1 | 2211.12 | 3499.80 | <0.0001 |
| B | 720.10 | 1 | 720.10 | 1139.79 | <0.0001 |
| C | 778.15 | 1 | 778.15 | 1231.67 | <0.0001 |
| AB | 35.40 | 1 | 35.40 | 56.04 | 0.0001 |
| AC | 80.10 | 1 | 80.10 | 126.79 | <0.0001 |
| BC | 1075.84 | 1 | 1075.84 | 1702.86 | <0.0001 |
| A2 | 3283.39 | 1 | 3283.39 | 5197.00 | <0.0001 |
| B2 | 717.06 | 1 | 717.06 | 1134.98 | <0.0001 |
| C2 | 262.78 | 1 | 262.78 | 415.93 | <0.0001 |
| Residual | 4.42 | 7 | 0.63 | ||
| Lack of Fit | 3.00 | 3 | 1.00 | 2.82 | 0.1712 |
| Pure Error | 1.42 | 4 | 0.35 | ||
| Cor Total | 9517.28 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lu, W.; Li, J.; Hu, J.; Ding, R.; Lv, M.; Wang, Q. Optimization of Ultrasonic-Assisted Extraction and Purification of Zeaxanthin and Lutein in Corn Gluten Meal. Molecules 2019, 24, 2994. https://doi.org/10.3390/molecules24162994
Wang L, Lu W, Li J, Hu J, Ding R, Lv M, Wang Q. Optimization of Ultrasonic-Assisted Extraction and Purification of Zeaxanthin and Lutein in Corn Gluten Meal. Molecules. 2019; 24(16):2994. https://doi.org/10.3390/molecules24162994
Chicago/Turabian StyleWang, Litao, Weihang Lu, Jiali Li, Jinxia Hu, Ruifang Ding, Mei Lv, and Qibao Wang. 2019. "Optimization of Ultrasonic-Assisted Extraction and Purification of Zeaxanthin and Lutein in Corn Gluten Meal" Molecules 24, no. 16: 2994. https://doi.org/10.3390/molecules24162994